Your Location:Home >Products >26787-75-7
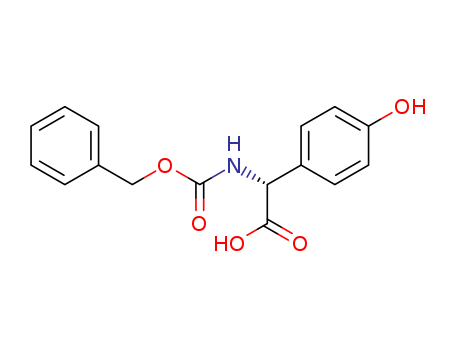

Product Details
β-Peptides are an interesting new class ...
Cp?Cobalt(III)-catalyzed enantioselectiv...
The present invention relates to compoun...
The invention discloses a D-hydroxy phen...
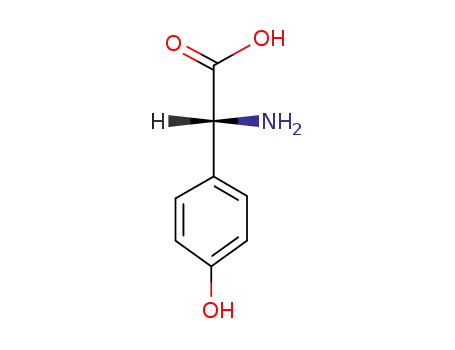
D-4-hydroxyphenylglycine

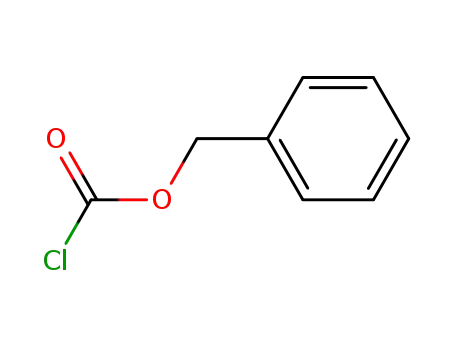
benzyl chloroformate

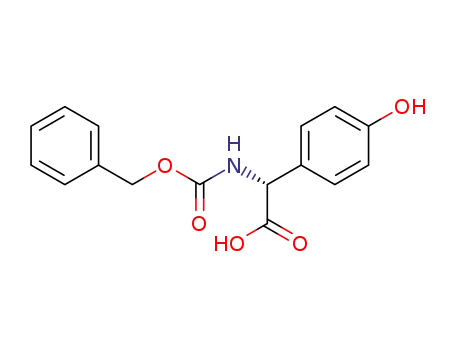
(R)-2-(((benzyloxy)carbonyl)amino)-2-(4-hydroxyphenyl)acetic acid
| Conditions | Yield |
|---|---|
|
With
sodium carbonate;
In
1,4-dioxane; water; toluene;
at 0 - 20 ℃;
for 1.5h;
|
93% |
|
With
sodium carbonate;
In
dichloromethane; water;
at 0 - 25 ℃;
|
88% |
|
With
sodium hydrogencarbonate;
In
water;
at 18 - 25 ℃;
for 2.33333h;
Inert atmosphere;
|
53% |
|
With
sodium hydrogencarbonate;
In
water;
for 1h;
|
|
|
With
potassium hydroxide;
In
water;
at 0 - 20 ℃;
for 0.0833333h;
|
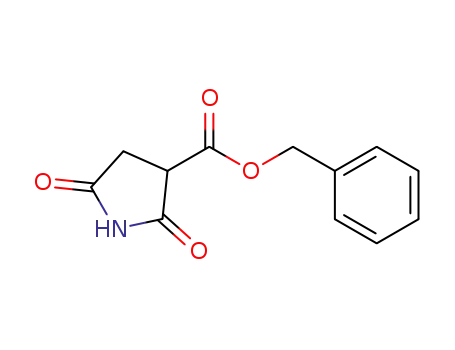
benzyloxycarbonyl succinimide


D-4-hydroxyphenylglycine


(R)-2-(((benzyloxy)carbonyl)amino)-2-(4-hydroxyphenyl)acetic acid
| Conditions | Yield |
|---|---|
|
With
potassium carbonate;
In
water; acetone;
at 15 - 35 ℃;
pH=8-9;
|
95% |

D-4-hydroxyphenylglycine

benzyl chloroformate
CAS:56-12-2
CAS:7531-52-4
CAS:74927-72-3