Your Location:Home >Products >Biochemical Engineering >7531-52-4
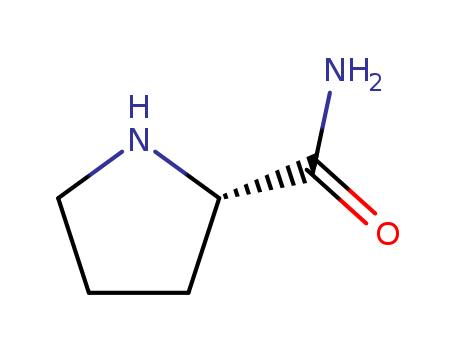

Product Details
|
Chemical Properties |
Crystalline |
|
Uses |
L-prolinamide is an important raw material and intermediate for pharmaceuticals, e.g. for antidiabetic drugs such as vildagliptin and agrochemicals and is also used as an organocatalyst. Combined with the use of L-prolinamide as a highly soluble and biochemically rather inert substance16 for competitive elution, Pro/Ala-rich sequences (PAS) affinity chromatography offers a mild procedure for the rapid and facile isolation of many different PASylated proteins. |
|
Definition |
ChEBI: The carboxamide derivative of L-proline. |
InChI:InChI=1/C5H10N2O/c6-5(8)4-2-1-3-7-4/h4,7H,1-3H2,(H2,6,8)/p+1/t4-/m1/s1
Considering the green aspects of chemistry in the context of developing eco-friendly catalysts, the small and easily accessible l-prolinamide molecules have been synthesized in high yields without using any complicated chemistry and expensive chemicals.
(L)‐Prolinamide containing imidazolium ionic liquids have been synthesized and evaluated as organocatalysts for the asymmetric Biginelli reaction of benzaldehydes, urea, and β‐keto …
In this study, bornyl- and cytisine-base...
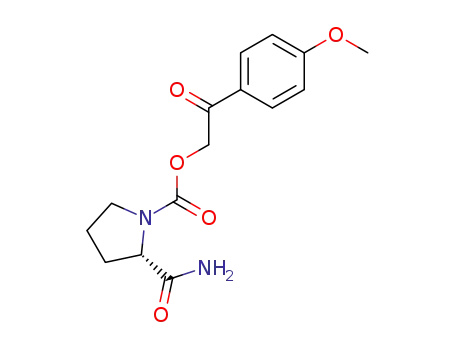
(S)-2-Carbamoyl-pyrrolidine-1-carboxylic acid 2-(4-methoxy-phenyl)-2-oxo-ethyl ester

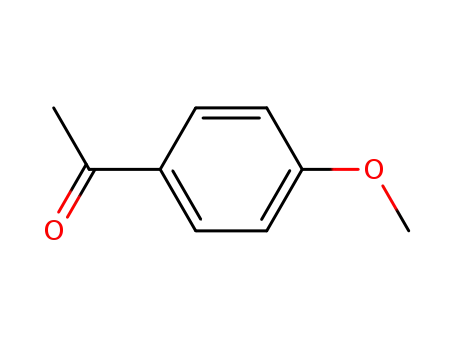
1-(4-methoxyphenyl)ethanone

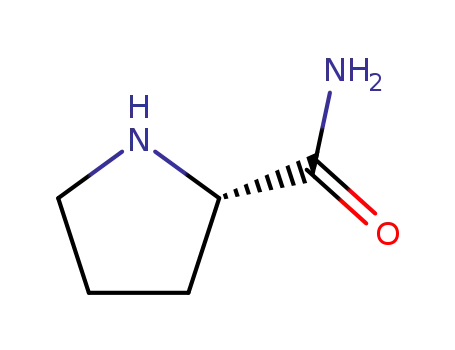
L-prolinamide
| Conditions | Yield |
|---|---|
|
Irradiation; mild conditions;
|
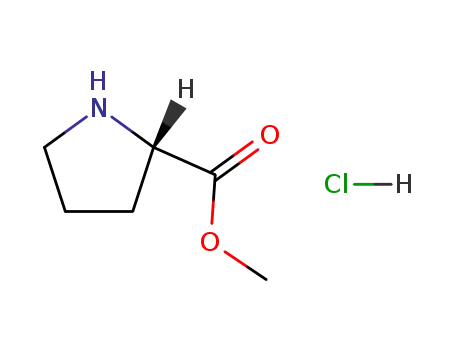
L-proline methyl ester monohydrochloride


L-prolinamide
| Conditions | Yield |
|---|---|
|
With ammonia; In methanol;
|
91% |
|
With ammonia; In methanol; at 0 - 20 ℃; for 15h; Reagent/catalyst; Large scale;
|
85% |
|
With ammonia; In methanol; at -25 ℃; for 96h; Sealed tube;
|
55% |
|
With ammonia; butan-1-ol; at 20 ℃; for 10h;
|
|
|
With ammonia; In methanol; Heating; Large scale;
|
18 kg |
|
With ammonia; at 0 ℃; for 26h; under 2250.23 Torr; Time;
|
15.8 g |
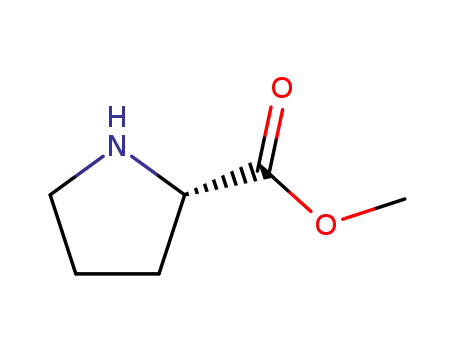
methyl (2S)-pyrrolidine carboxylate
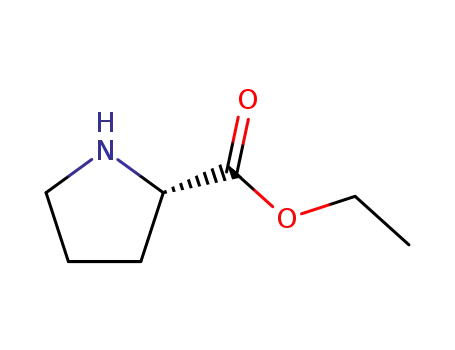
ethyl (2S)-pyrrolidine-2-carboxylate

(S)-2-Carbamoyl-pyrrolidine-1-carboxylic acid 2-(4-methoxy-phenyl)-2-oxo-ethyl ester
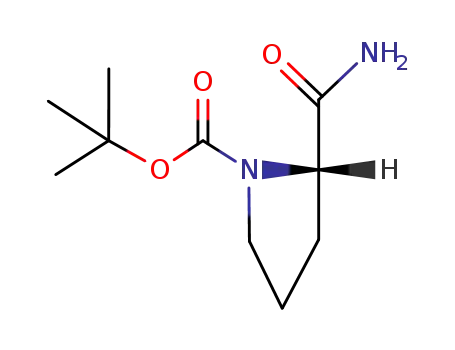
(S)-tert-butyl 2-carbamoylpyrrolidine-1-carboxylate
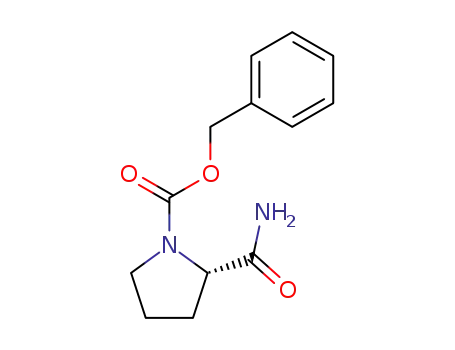
Z-Pro-NH2
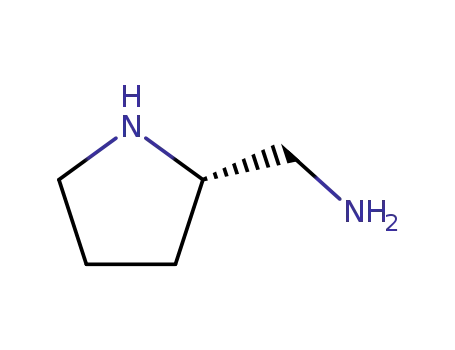
(S)-2-Aminomethylpyrrolidin
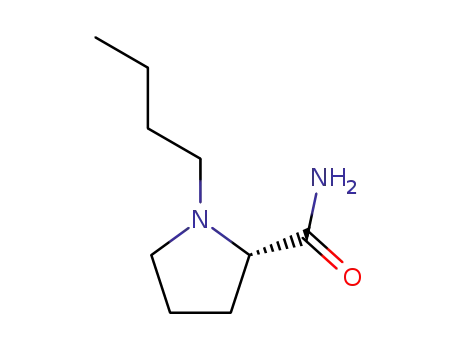
1-nbutylprolinamide
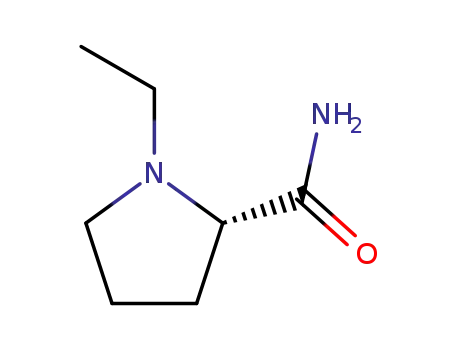
(-)-(S)-1-ethyl-2-pyrrolidinecarboxamide
CAS:56-12-2
CAS:98-79-3
CAS:47375-34-8
CAS:84793-07-7