Your Location:Home >Products >Biochemical Engineering >21149-17-7
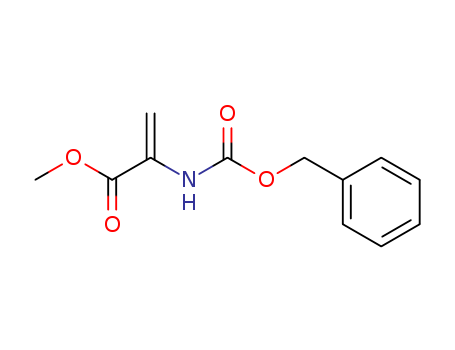

Product Details
|
Chemical Properties |
White solid |
|
Synthesis Reference(s) |
Canadian Journal of Chemistry, 59, p. 406, 1981 DOI: 10.1139/v81-062The Journal of Organic Chemistry, 31, p. 3928, 1966Synthetic Communications, 19, p. 695, 1989 DOI: 10.1080/00397918908050717 |
InChI:InChI=1/C12H13NO4/c1-9(11(14)16-2)13-12(15)17-8-10-6-4-3-5-7-10/h3-7H,1,8H2,2H3,(H,13,15)
A novel solid-phase synthetic method for...
3-Imidoallenylphosphonates, allenes bear...
ABSTRACT: Not only α,β-dehydroamino acid...
Suitably protected amino acids were used...
Provided is pharmaceutical composition f...
The present invention relates to a metho...
This report discloses the first example ...
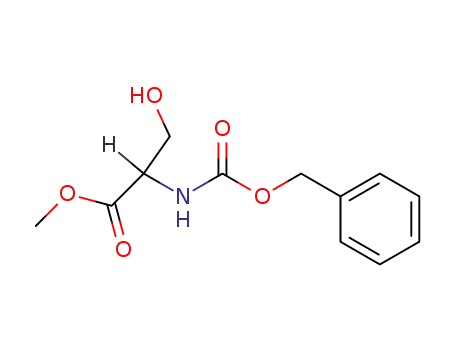
methyl 2-(benzyloxycarbonylamino)-3-hydroxypropanoate

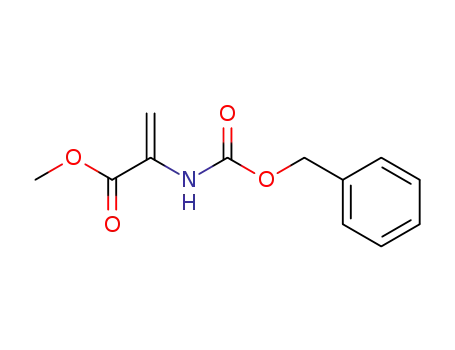
methyl 2-(benzyloxycarbonylamino)acrylate
| Conditions | Yield |
|---|---|
|
With
dmap; 1,8-diazabicyclo[5.4.0]undec-7-ene; p-toluenesulfonyl chloride;
In
acetonitrile;
at 20 ℃;
Molecular sieve;
|
72% |
|
methyl 2-(benzyloxycarbonylamino)-3-hydroxypropanoate;
With
dichloroacethyl chloride; triethylamine;
In
dichloromethane;
at 20 ℃;
for 1.5h;
With
1,8-diazabicyclo[5.4.0]undec-7-ene;
In
dichloromethane;
for 4h;
Heating;
|
48% |
|
Multi-step reaction with 2 steps
1: 81 percent / pyridine / CH2Cl2 / 2 h / 20 °C
2: 84 percent / K2CO3 / dimethylformamide / 1 h / 65 °C
With
pyridine; potassium carbonate;
In
dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: 58 percent / phosphorus pentachloride / CHCl3 / 4 h / 0 °C
2: 84 percent / diazabicyclo<5.4.0>undec-7-ene / CHCl3 / 1 h / 60 °C
With
phosphorus pentachloride; 1,8-diazabicyclo[5.4.0]undec-7-ene;
In
chloroform;
|
|
|
With
methanesulfonyl chloride; triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 1.33333h;
|
1.1 g |
|
Multi-step reaction with 2 steps
1.1: triethylamine / tetrahydrofuran / 0.17 h / 0 °C / Inert atmosphere
1.2: 1 h / 0 °C
2.1: triethylamine / tetrahydrofuran / 3 h / 0 - 20 °C / Inert atmosphere
With
triethylamine;
In
tetrahydrofuran;
|
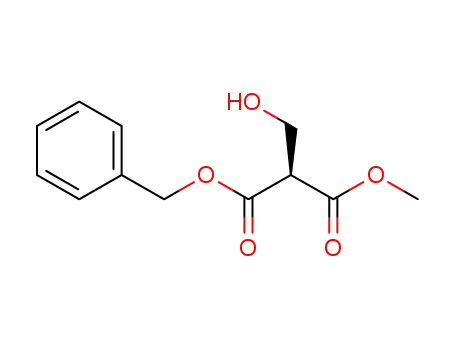
(S)-methyl 2-(benzyloxycarbonyl)-3-hydroxypropanoate

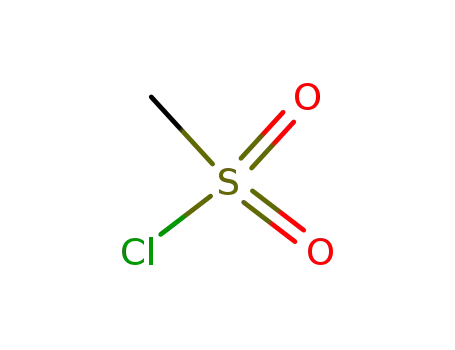
methanesulfonyl chloride


methyl 2-(benzyloxycarbonylamino)acrylate
| Conditions | Yield |
|---|---|
|
(S)-methyl 2-(benzyloxycarbonyl)-3-hydroxypropanoate; methanesulfonyl chloride;
With
triethylamine;
In
dichloromethane;
at -15 - 0 ℃;
for 0.5h;
With
methanol;
In
dichloromethane;
at 20 ℃;
for 1.5h;
|
92% |

diazomethane
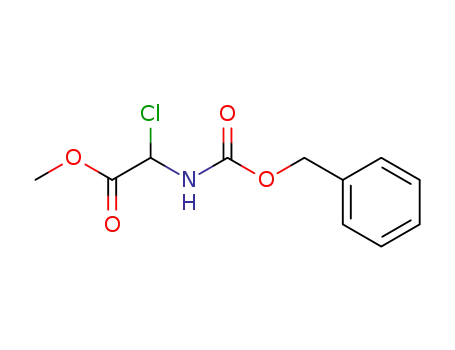
N-benzyloxycarbonyl α-chloro glycine methyl ester
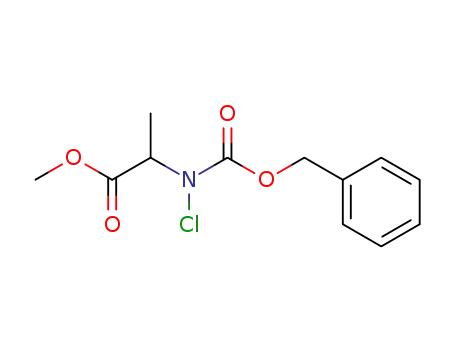
C12H14ClNO4
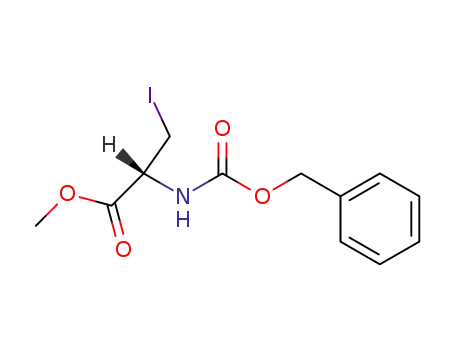
methyl N-((benzyloxy)carbonyl)-3-iodo-L-alaninate
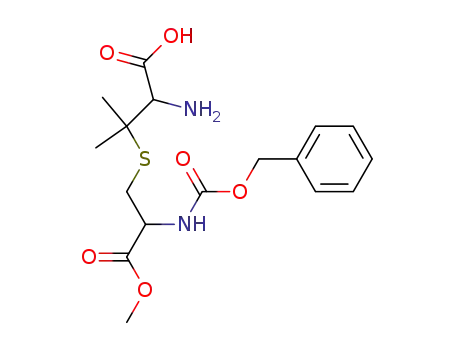
S-(2-benzyloxycarbonylamino-2-methoxycarbonyl-ethyl)-penicillamine
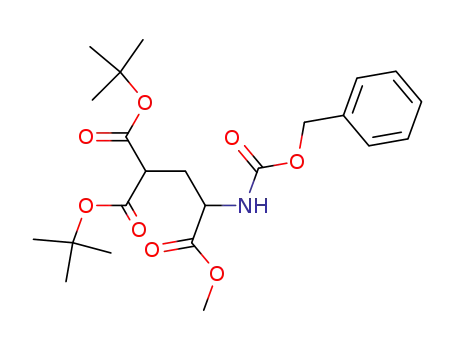
Z-γ,γ,-di-tert-butyl-D,L-carboxyglutamic acid methyl ester
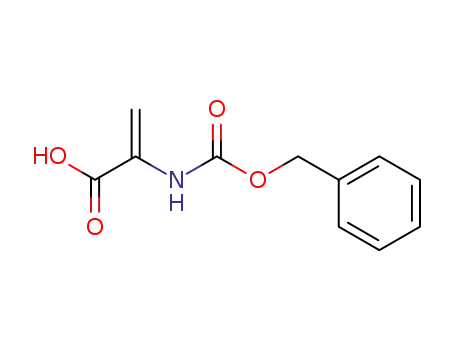
2-benzyloxycarbonylamino-acrylic acid
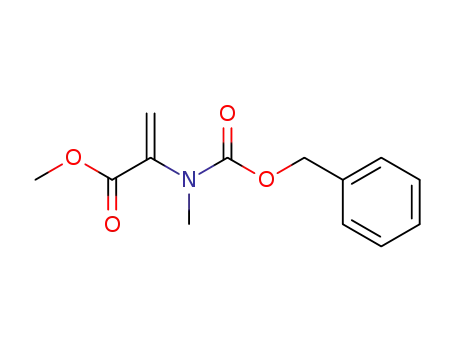
2-(Benzyloxycarbonyl-methyl-amino)-acrylic acid methyl ester
CAS:56-12-2
CAS:7531-52-4
CAS:955379-18-7
CAS:124668-49-1