Your Location:Home >Products >124668-49-1
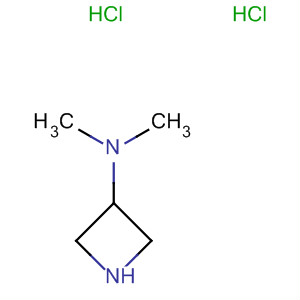

Product Details
InChI:InChI=1/C5H12N2/c1-7(2)5-3-6-4-5/h5-6H,3-4H2,1-2H3
A cyclic amine derivative represented by...
The present invention relates to 2,3,4,6...
The present invention relates to a 2,4-d...
The present invention relates to certain...
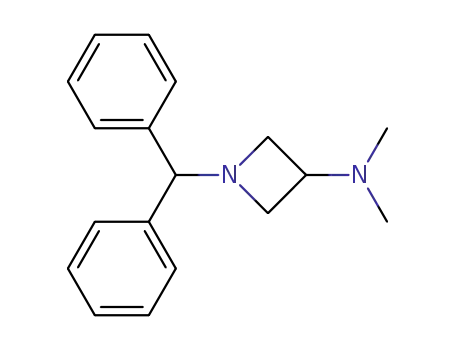
3-(dimethylamino)-1-(diphenylmethyl)azetidine

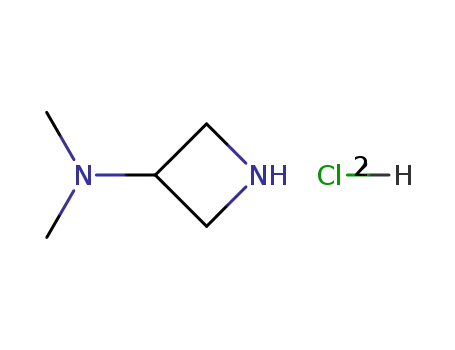
3-(dimethylamino)azetidine dihydrochloride
| Conditions | Yield |
|---|---|
|
3-(dimethylamino)-1-(diphenylmethyl)azetidine;
With
hydrogen;
palladium(II) hydroxide/carbon;
In
ethanol;
for 18h;
With
hydrogenchloride;
In
ethanol;
|
87% |
|
3-(dimethylamino)-1-(diphenylmethyl)azetidine;
With
hydrogen; dimethyl amine;
palladium(II) hydroxide/carbon;
In
ethanol;
for 18h;
With
hydrogenchloride;
In
ethanol;
|
87% |
|
With
hydrogenchloride; hydrogen;
palladium(II) hydroxide/carbon;
In
ethanol;
for 18h;
|
87% |
|
With
hydrogenchloride; hydrogen;
palladium on activated charcoal;
In
methanol;
|
|
|
With
hydrogenchloride; palladium dihydroxide; hydrogen;
Multistep reaction;
1) Et2O, 0 deg C; 2) EtOH, 60psi, r.t.;
|
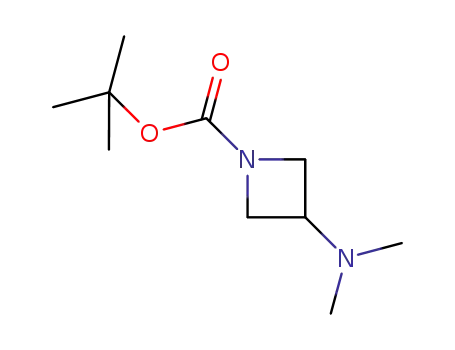
tert-butyl 3-(dimethylamino)azetidine-1-carboxylate


3-(dimethylamino)azetidine dihydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
1,4-dioxane; dichloromethane;
at 20 ℃;
for 3h;
|
82% |
|
With
hydrogenchloride;
In
1,4-dioxane;
at 0 - 20 ℃;
for 3h;
|
82% |

3-(dimethylamino)-1-(diphenylmethyl)azetidine

tert-butyl 3-(dimethylamino)azetidine-1-carboxylate
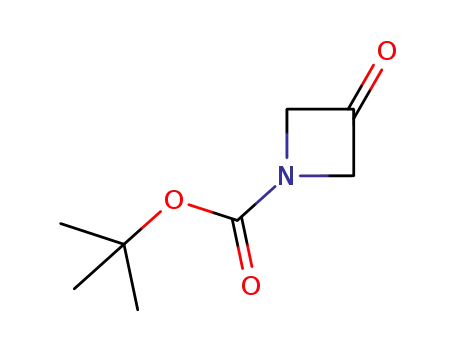
tert-butyl 3-oxoazetidine-1-carboxylate
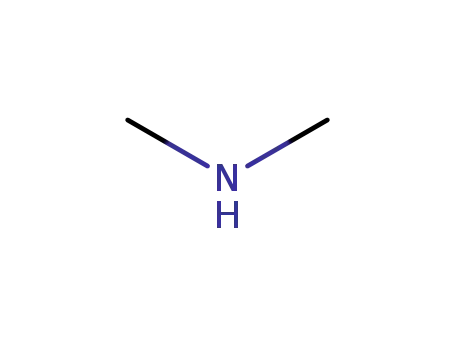
dimethyl amine
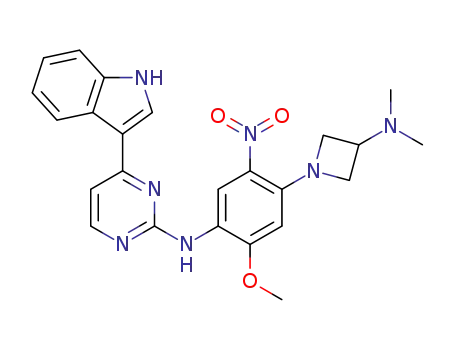
N-[4-(3-dimethylaminoazetidin-1-yl)-2-methoxy-5-nitrophenyl]-4-(1H-indol-3-yl)pyrimidin-2-amine
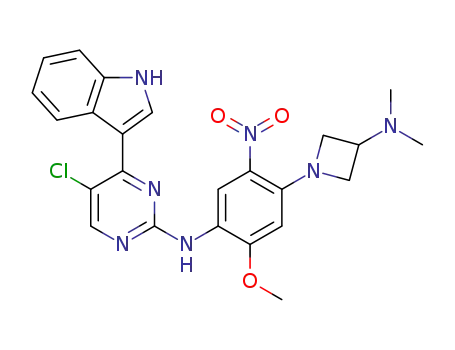
5-chloro-N-[4-(3-dimethylaminoazetidin-1-yl)-2-methoxy-5-nitrophenyl]-4-(1H-indol-3-yl)pyrimidin-2-amine
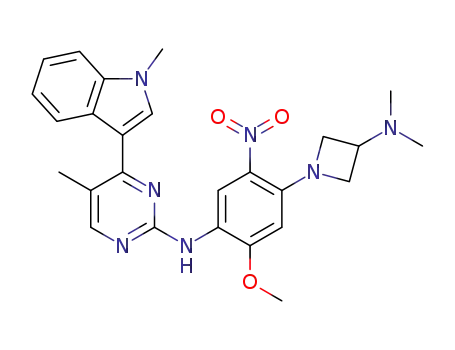
N-[4-(3-dimethylaminoazetidin-1-yl)-2-methoxy-5-nitrophenyl]-5-methyl-4-(1-methylindol-3-yl)pyrimidin-2-amine
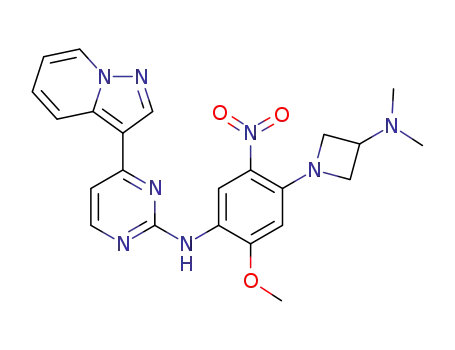
N-[4-(3-dimethylaminoazetidin-1-yl)-2-methoxy-5-nitrophenyl]-4-pyrazolo[1,5-a]pyridin-3-ylpyrimidin-2-amine
CAS:138-15-8
CAS:3196-73-4
CAS:21149-17-7
CAS:51154-06-4