Your Location:Home >Products >Biochemical Engineering >51154-06-4
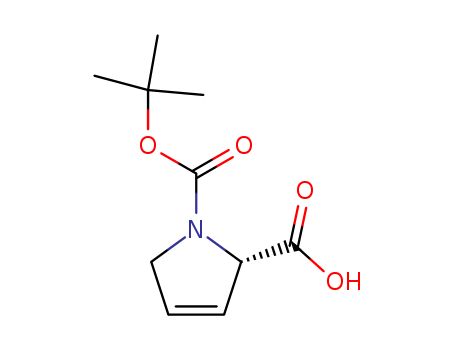

Product Details
|
Chemical Properties |
White to off-white crystalline powder |
|
Uses |
Boc-3,4-Dehydro-L-Proline is an N-terminal protected 3,4-Dehydro-L-proline. It is used in solid-phase peptide synthesis (SPPS) to make peptides. 3,4-Dehydro-L-proline is a alternate substrate of the amino acid oxidase, NikD. |
|
Biochem/physiol Actions |
Boc-3,4-Dehydro-L-Proline is an N-terminal protected amino acid used in solid-phase peptide synthesis (SPPS) to make peptides containing 3,4-Dehydro-L-proline. |
Based on the observation that an increas...
Disclosed is a method for efficiently pr...
Starting from 2-pyrrolecarboxylic acid t...
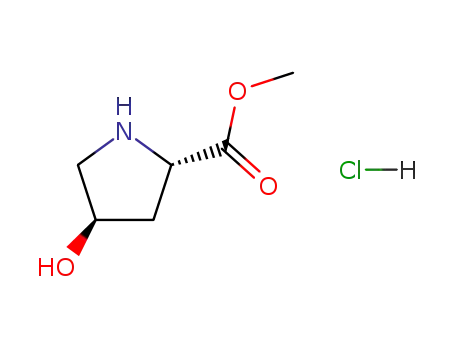
L-4-hydroxyproline methyl ester hydrochloride

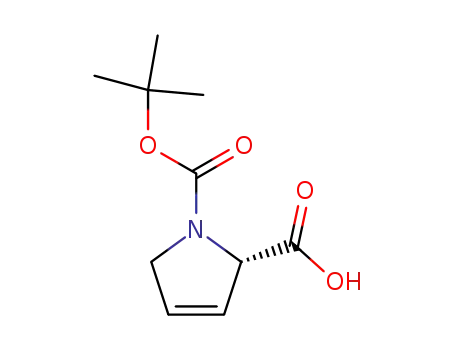
(S)-2,5-dihydropyrrole-1,2-dicarboxylic acid 1-tert-butyl ester
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 5 steps
1: dmap; triethylamine / dichloromethane / 19 h / 0 - 20 °C
2: dmap; triethylamine / dichloromethane / 5 h / 0 - 20 °C
3: sodium tetrahydroborate / tert-butyl alcohol / 80 °C
4: pyridine; dihydrogen peroxide / water; dichloromethane / 3 h / 20 °C
5: lithium hydroxide / tetrahydrofuran; water / 2 h / 20 °C
With
pyridine; dmap; sodium tetrahydroborate; dihydrogen peroxide; triethylamine; lithium hydroxide;
In
tetrahydrofuran; dichloromethane; water; tert-butyl alcohol;
|
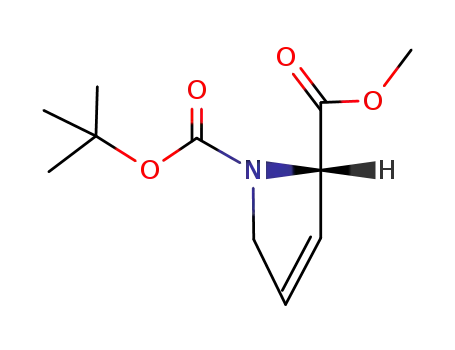
N-BOC-3,4-didehydro-(S)-proline methyl ester

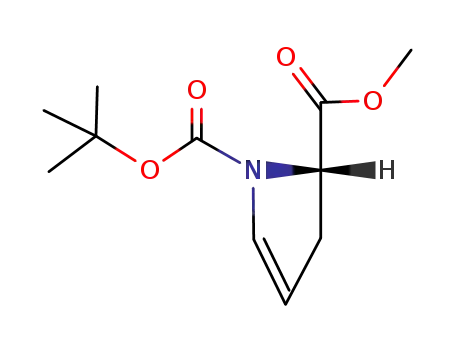
(S)-1-tert-butyl 2-methyl 2,3-dihydro-1H-pyrrole-1,2-dicarboxylate

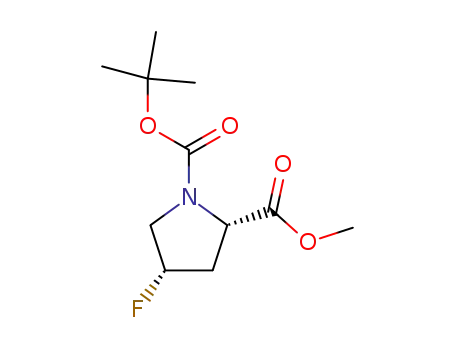
1-tert-butyl 2-methyl (2S,4S)-4-fluoropyrrolidine-1,2-dicarboxylate

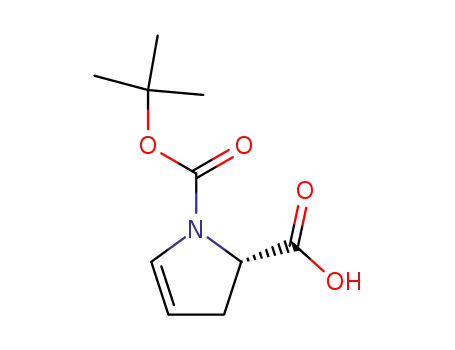
1-(tert-butoxycarbonyl)-2,3-dihydro-1H-pyrrole-2-carboxylic acid


(S)-2,5-dihydropyrrole-1,2-dicarboxylic acid 1-tert-butyl ester

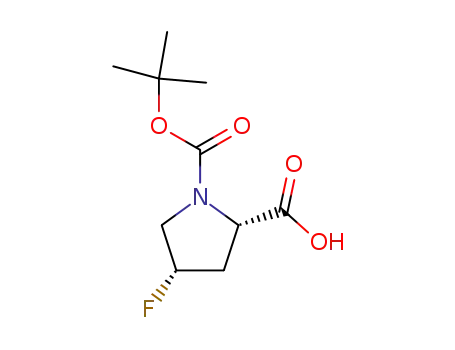
(2S,4S)-1-(tert-butoxycarbonyl)-4-fluoropyrrolidine-2-carboxylic acid
| Conditions | Yield |
|---|---|
|
N-BOC-3,4-didehydro-(S)-proline methyl ester; (S)-1-tert-butyl 2-methyl 2,3-dihydro-1H-pyrrole-1,2-dicarboxylate; 1-tert-butyl 2-methyl (2S,4S)-4-fluoropyrrolidine-1,2-dicarboxylate;
With
sodium hydroxide; water;
at 40 ℃;
for 2h;
With
hydrogenchloride;
In
water;
at 5 ℃;
pH=5;
With
hydrogenchloride; sodium hydroxide; sodium hypochlorite;
more than 3 stages;
Product distribution / selectivity;
|
0.01 %Chromat. 0.01 %Chromat. |
|
N-BOC-3,4-didehydro-(S)-proline methyl ester; (S)-1-tert-butyl 2-methyl 2,3-dihydro-1H-pyrrole-1,2-dicarboxylate; 1-tert-butyl 2-methyl (2S,4S)-4-fluoropyrrolidine-1,2-dicarboxylate;
With
sodium hydroxide; water;
at 40 ℃;
for 2h;
With
hydrogenchloride;
In
water;
pH=2;
Product distribution / selectivity;
|
1 %Chromat. 0.2 %Chromat. |
|
N-BOC-3,4-didehydro-(S)-proline methyl ester; (S)-1-tert-butyl 2-methyl 2,3-dihydro-1H-pyrrole-1,2-dicarboxylate; 1-tert-butyl 2-methyl (2S,4S)-4-fluoropyrrolidine-1,2-dicarboxylate;
With
bromine;
In
dichloromethane;
at 5 - 20 ℃;
for 4h;
With
sodium hydroxide; water;
In
methanol;
at 40 ℃;
for 2h;
With
hydrogenchloride;
In
methanol; water;
pH=2;
Product distribution / selectivity;
|
0.02 %Chromat. 0.01 %Chromat. |
|
N-BOC-3,4-didehydro-(S)-proline methyl ester; (S)-1-tert-butyl 2-methyl 2,3-dihydro-1H-pyrrole-1,2-dicarboxylate; 1-tert-butyl 2-methyl (2S,4S)-4-fluoropyrrolidine-1,2-dicarboxylate;
With
sodium hydroxide; water;
at 40 ℃;
for 2h;
With
hydrogenchloride;
In
water;
at 5 ℃;
pH=5;
With
hydrogenchloride; sodium hydroxide; N-Bromosuccinimide;
more than 3 stages;
Product distribution / selectivity;
|
0.01 %Chromat. 0.01 %Chromat. |
|
N-BOC-3,4-didehydro-(S)-proline methyl ester; (S)-1-tert-butyl 2-methyl 2,3-dihydro-1H-pyrrole-1,2-dicarboxylate; 1-tert-butyl 2-methyl (2S,4S)-4-fluoropyrrolidine-1,2-dicarboxylate;
With
sodium hydroxide; water;
at 40 ℃;
for 2h;
With
hydrogenchloride;
In
water;
at 5 ℃;
pH=5;
With
hydrogenchloride; sodium hydroxide; bromine;
more than 3 stages;
Product distribution / selectivity;
|
0.01 %Chromat. 0.01 %Chromat. |

N-BOC-3,4-didehydro-(S)-proline methyl ester

(S)-1-tert-butyl 2-methyl 2,3-dihydro-1H-pyrrole-1,2-dicarboxylate

1-tert-butyl 2-methyl (2S,4S)-4-fluoropyrrolidine-1,2-dicarboxylate

L-4-hydroxyproline methyl ester hydrochloride
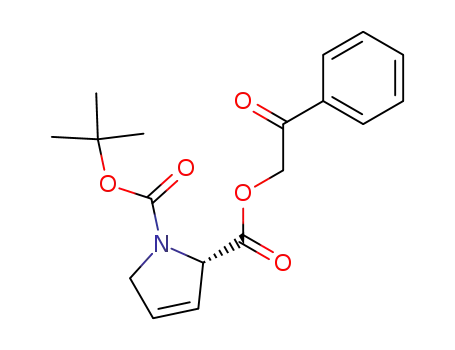
Boc-3,4-dehydro-L-proline phenacyl ester
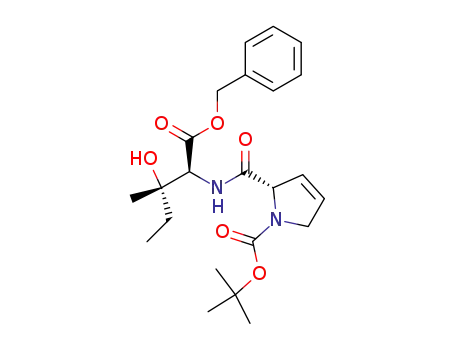
(S)-2-((1S,2S)-1-Benzyloxycarbonyl-2-hydroxy-2-methyl-butylcarbamoyl)-2,5-dihydro-pyrrole-1-carboxylic acid tert-butyl ester
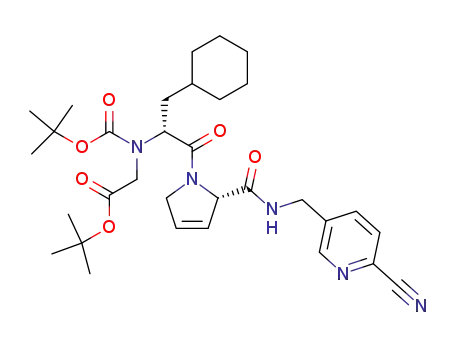
tert-butyl {(tert-butoxycarbonyl)[(1R)-2-[(2S)-2-({[(6-cyano-3-pyridinyl)methyl]amino}carbonyl)-2,5-dihydro-1H-pyrrol-1-yl]-1-(cyclohexylmethyl)-2-oxoethyl]amino}acetate
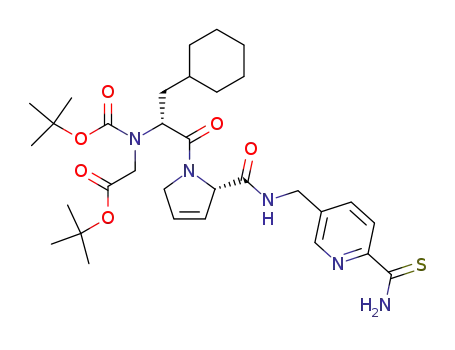
[tert-butoxycarbonyl-(1-cyclohexylmethyl-2-oxo-2-{2-[(6-thiocarbamoyl-pyridin-3-ylmethyl)-carbamoyl]-2,5-dihydro-pyrrol-1-yl}-ethyl)-amino]-acetic acid tert-butyl ester
CAS:56-12-2
CAS:7531-52-4
CAS:124668-49-1
CAS:2566-30-5