Your Location:Home >Products >Biochemical Engineering >3196-73-4
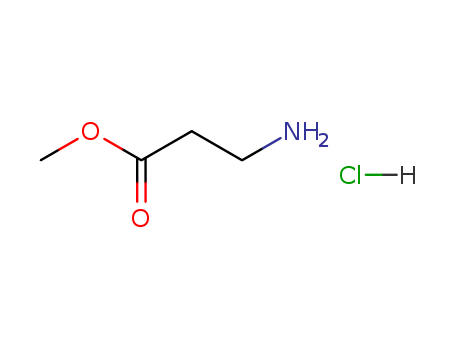

Product Details
|
Chemical Properties |
white to off-white crystalline powder |
|
Uses |
β-Alanine Methyl Ester is an intermediate in the synthesis of Trichostatin A (T774710) and Trapoxin B as histone deacetylase inhibitors. b-Alanine methyl ester hydrochloride is a fatty acid that is found in animal and plant tissues. |
Isomeric SMILES: COC(=O)CCN.Cl
InChIKey: XPGRZDJXVKFLHQ-UHFFFAOYSA-N
InChI: InChI=1S/C4H9NO2.ClH/c1-7-4(6)2-3-5;/h2-3,5H2,1H3;1H
Briefly, a suspension of 2-fluoro-,B-alanine methyl ester hydrochloride (0.5 mmol) in 7 ml of ethyl acetate containing 0.1 ml oftriethylamine was stirred at 25Cfor 30 min, after which cholic …
… protected by conversion into methyl ester II. The reaction was performed in methanol in the presence of thionyl chloride [6]. The amino group of b-alanine methyl ester hydrochloride was …
A new three-residue turn in β peptides n...
methanol

3-amino propanoic acid

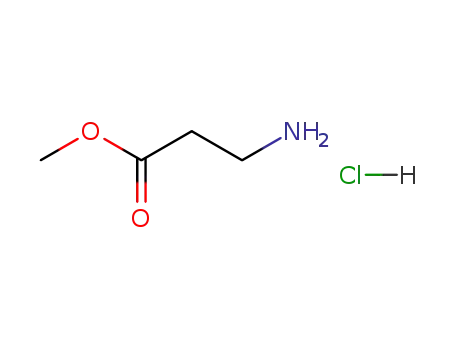
methyl 3-aminopropanoate hydrochloride
| Conditions | Yield |
|---|---|
|
methanol; With thionyl chloride; for 1h; Cooling with ice;
3-amino propanoic acid; at 20 - 66 ℃; for 6.5h;
|
100% |
|
With thionyl chloride; for 3h; Reflux;
|
100% |
|
With thionyl chloride; at 0 - 20 ℃;
|
99% |
|
With thionyl chloride; at 0 - 20 ℃; for 2h; Cooling with ice;
|
98% |
|
With chloro-trimethyl-silane; for 6h; Ambient temperature;
|
96% |
|
With thionyl chloride; Heating;
|
96% |
|
With chloro-trimethyl-silane; at 20 ℃; for 12h;
|
96% |
|
With thionyl chloride; at 0 ℃; for 1h;
|
91% |
|
With acetyl chloride; at 0 ℃; for 3h; Reflux;
|
91% |
|
With chloro-trimethyl-silane; at 0 - 20 ℃;
|
90% |
|
With thionyl chloride; at 20 ℃; for 18h;
|
89% |
|
With thionyl chloride; for 2.5h;
|
88% |
|
With thionyl chloride; at 0 ℃;
|
85% |
|
With thionyl chloride; at 0 - 20 ℃;
|
78% |
|
With thionyl chloride; at 20 ℃; for 16h;
|
77% |
|
methanol; With thionyl chloride; at 0 ℃; for 0.333333h;
3-amino propanoic acid; at 20 ℃; for 14h;
|
62% |
|
With hydrogenchloride;
|
|
|
With hydrogenchloride; for 17h; Heating;
|
|
|
With hydrogenchloride; at 60 ℃; for 1.5h;
|
|
|
With thionyl chloride; for 12h; Heating;
|
|
|
With thionyl chloride;
|
|
|
With hydrogenchloride;
|
|
|
With thionyl chloride; at 60 - 70 ℃;
|
|
|
With thionyl chloride; at 5 ℃; Inert atmosphere; Reflux;
|
|
|
With thionyl chloride; at 78 ℃;
|
|
|
With hydrogenchloride; at 20 ℃;
|
|
|
With thionyl chloride; Inert atmosphere; Reflux;
|
|
|
methanol; With thionyl chloride; at 0 ℃; for 1h;
3-amino propanoic acid; at 90 ℃; for 15h;
|
|
|
With hydrogenchloride; at 20 ℃;
|
|
|
With thionyl chloride; at 0 - 20 ℃; for 16h; Inert atmosphere;
|
|
|
With thionyl chloride; at 70 ℃; for 16h;
|
|
|
With thionyl chloride; at 60 ℃; Cooling with ice;
|
|
|
With thionyl chloride;
|
|
|
With thionyl chloride; at 40 ℃; for 12h;
|
|
|
With thionyl chloride;
|
|
|
With thionyl chloride; for 12h; Reflux;
|
|
|
With thionyl chloride; for 5h; Inert atmosphere; Reflux;
|
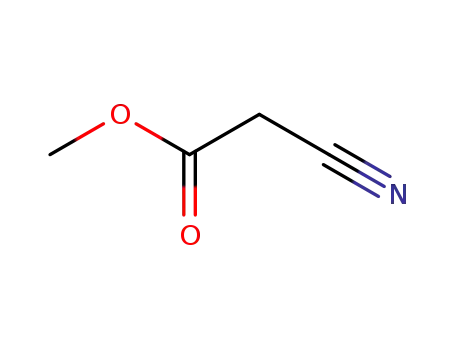
methyl 2-cyanoacetate


methyl 3-aminopropanoate hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogen; potassium hydroxide; In methanol; at 30 ℃; for 2h; under 15001.5 Torr;
|
98.6% |

methanol

3-amino propanoic acid
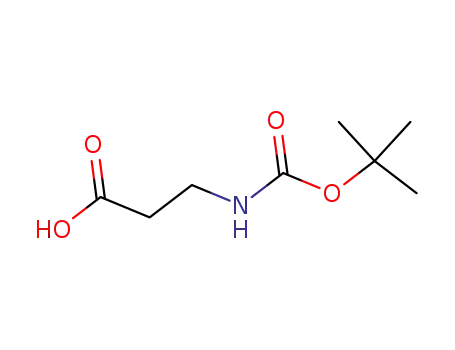
3-(tert-butyloxycarbonylamino)propionic acid
3-<(tert-butoxycarbonyl)amino>propionitrile

3-amino propanoic acid
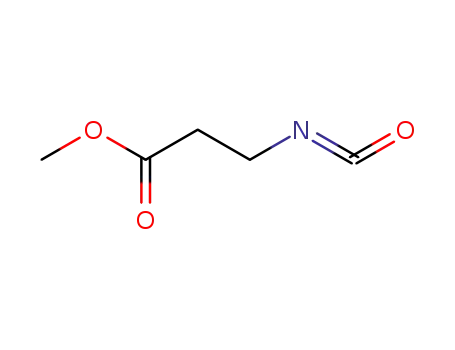
2-methoxycarbonylethyl isocyanate
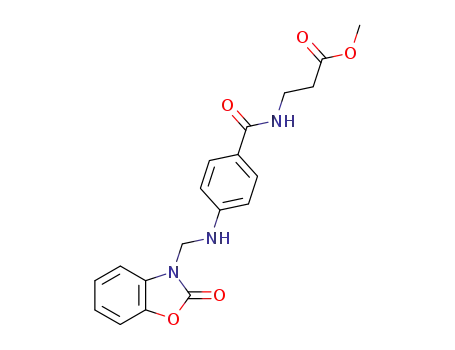
N-{4-[(2-oxo-benzooxazol-3-ylmethyl)-amino]-benzoyl}-β-alanine methyl ester
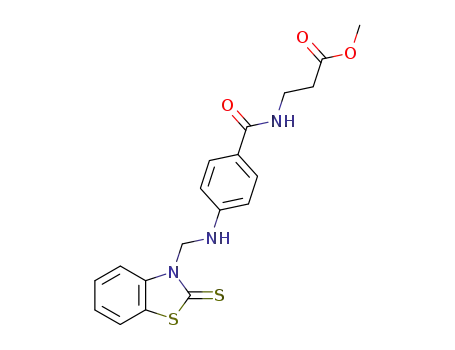
N-{4-[(2-thioxo-benzothiazol-3-ylmethyl)-amino]-benzoyl}-β-alanine methyl ester
CAS:138-15-8
CAS:4244-84-2
CAS:2462-32-0