Your Location:Home >Products >Biochemical Engineering >2462-32-0
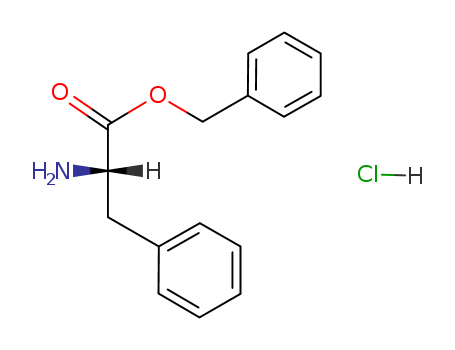

Product Details
|
Chemical Properties |
White crystal |
|
Uses |
L-Phenylalanine Benzyl Ester Hydrochloride is a commonly used reactant for the synthesis of L-isoserine derivatives as aminopeptidase N inhibitors and PF-04449913 as a potent and orally bioavailable inhibitor of smoothened. |
InChI:InChI=1/C16H17NO2.ClH/c17-15(11-13-7-3-1-4-8-13)16(18)19-12-14-9-5-2-6-10-14;/h1-10,15H,11-12,17H2;1H/t15-;/m0./s1
The present application relates to novel...
Chiral recognition is based on a large n...
A series of novel L-isoserine derivative...
Trypanosoma cruzi and Trypanosoma brucei...
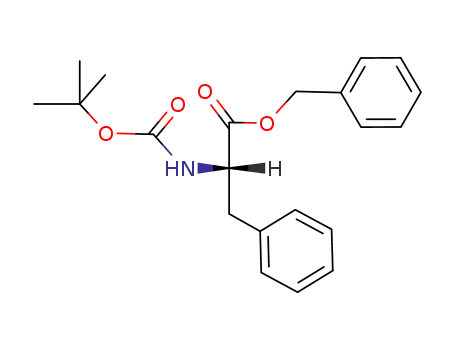
N-(tert-butoxycarbonyl)-L-phenylalanine benzyl ester

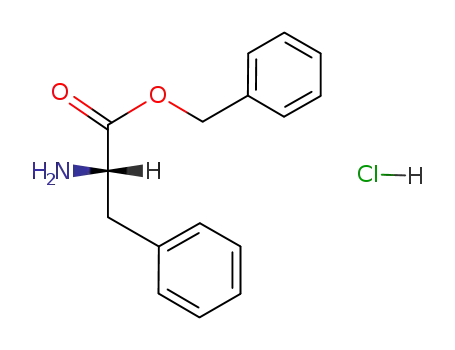
L-phenylalanine benzyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
1,4-dioxane;
at 20 ℃;
Inert atmosphere;
|
100% |
|
With
hydrogenchloride;
In
1,4-dioxane; diethyl ether;
at 20 ℃;
|
76% |
|
With
hydrogenchloride;
In
1,4-dioxane;
|
|
|
With
hydrogenchloride;
In
ethyl acetate;
|
|
|
With
hydrogenchloride;
In
tetrahydrofuran; 1,4-dioxane;
at 0 ℃;
for 12h;
Inert atmosphere;
|
hydrochloric acid-dioxane


N-(tert-butoxycarbonyl)-L-phenylalanine benzyl ester


L-phenylalanine benzyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
In
diethyl ether; chloroform;
|
94% |
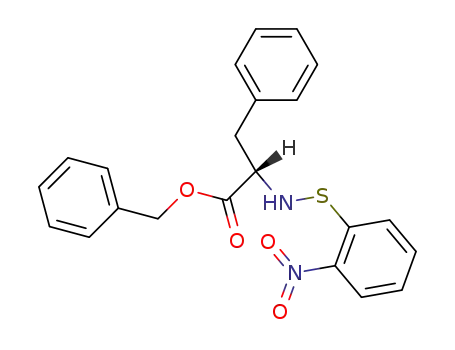
(S)-2-(2-Nitro-phenylsulfanylamino)-3-phenyl-propionic acid benzyl ester
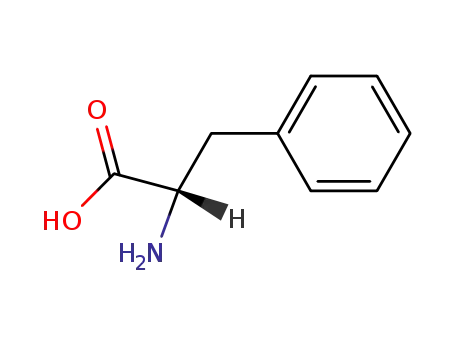
L-phenylalanine
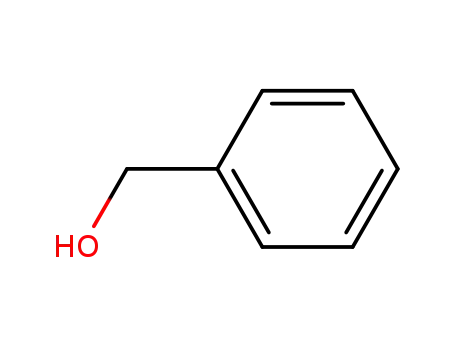
benzyl alcohol

N-(tert-butoxycarbonyl)-L-phenylalanine benzyl ester
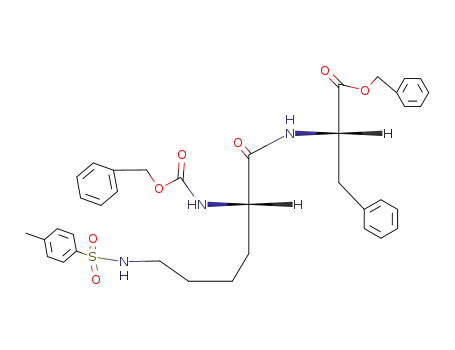
Carbobenzoxy-(Nε-tosyl)-lysyl-phenylalaninbenzylester
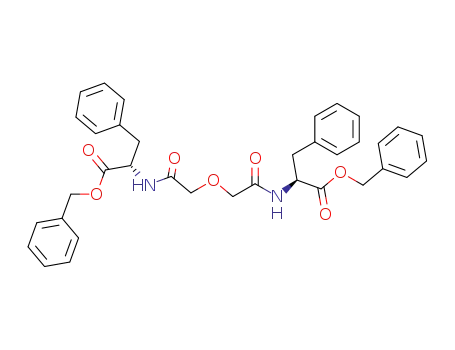
(S)-2-{2-[((S)-1-Benzyloxycarbonyl-2-phenyl-ethylcarbamoyl)-methoxy]-acetylamino}-3-phenyl-propionic acid benzyl ester
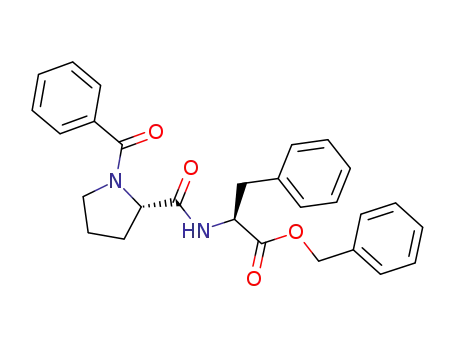
N-benzoyl-L-prolyl-L-phenylalanine benzyl ester
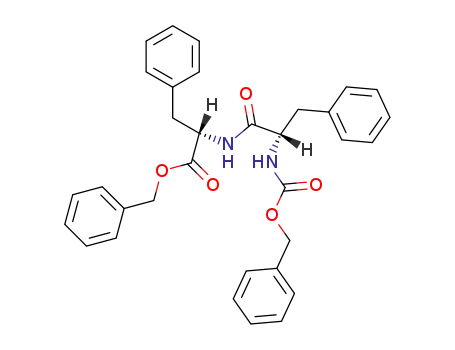
(S)-benzyl 2-((S)-2-(((benzyloxy)carbonyl)amino)-3-phenylpropanamido)-3-phenylpropanoate
CAS:138-15-8
CAS:3196-73-4
CAS:4244-84-2
CAS:7524-50-7