Your Location:Home >Products >Biochemical Engineering >7524-50-7
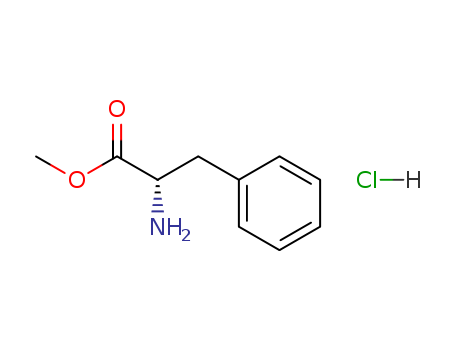

Product Details
|
Chemical Properties |
white to off-white fine crystalline powder |
|
Uses |
Protected (S) enantiomer of the amino acid Phenylalanine. |
InChI:InChI=1/C10H13NO2/c1-13-10(12)9(11)7-8-5-3-2-4-6-8/h2-6,9H,7,11H2,1H3/p+1/t9-/m0/s1
Two different types of new phosphinamide...
The invention provides application of a ...
New C2 symmetric chiral diimines were sy...
Elaborate fragments of the proposed ster...
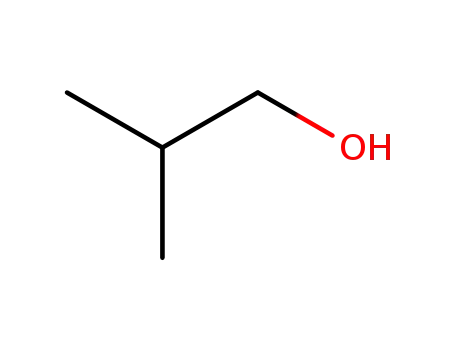
2-methyl-propan-1-ol

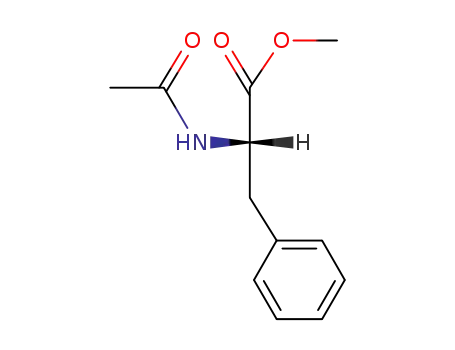
(S)-N-acetylphenylalanine

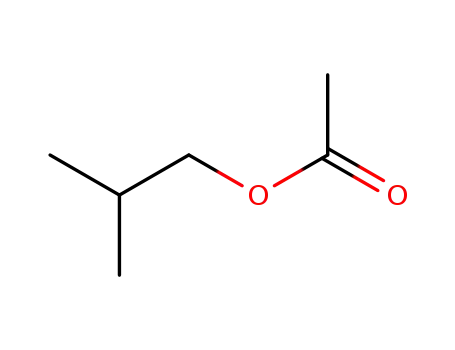
2-methylpropyl acetate

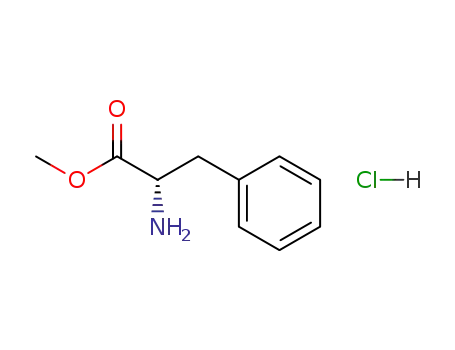
methyl (2S)-2-amino-3-phenylpropanoate hydrochloride
| Conditions | Yield |
|---|---|
|
(S)-N-acetylphenylalanine;
With
triphenyl phosphite; chlorine; triethylamine;
In
tetrahydrofuran;
at -30 ℃;
2-methyl-propan-1-ol;
In
tetrahydrofuran;
at -30 - 20 ℃;
With
water;
|
95% |
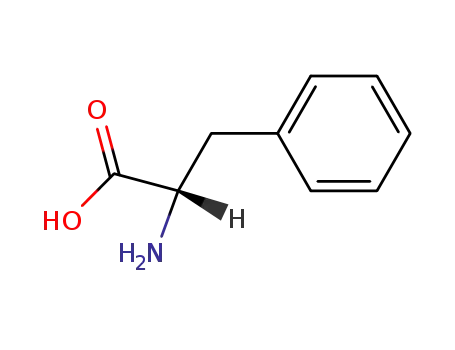
L-phenylalanine


methyl (2S)-2-amino-3-phenylpropanoate hydrochloride
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
In
methanol;
|
100% |
|
With
thionyl chloride;
In
methanol;
at 0 - 20 ℃;
|
100% |
|
With
thionyl chloride;
In
methanol;
|
98% |
|
With
thionyl chloride;
In
methanol; diethyl ether;
|
95% |
|
In
methanol; benzene;
for 4h;
Ambient temperature;
|
42% |
|
With
hydrogenchloride;
|
|
|
Multi-step reaction with 2 steps
1: thionyl chloride
With
thionyl chloride;
|
|
|
With
hydrogenchloride; methanol;
In
di-isopropyl ether;
at 55 ℃;
for 0.0833333h;
Heating / reflux;
|
|
|
With
thionyl chloride;
In
methanol;
|
|
|
With
hydrogenchloride;
In
methanol; toluene;
|
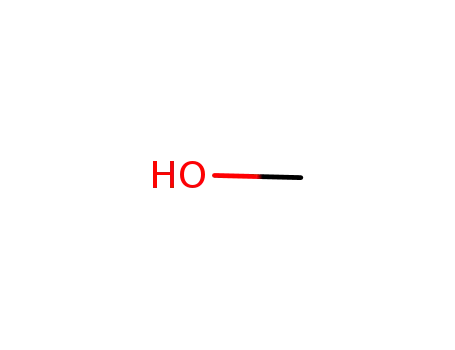
methanol

L-phenylalanine
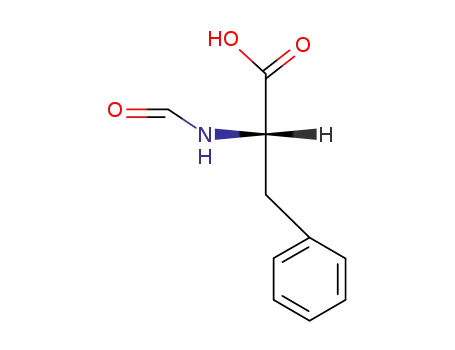
N-formyl-L-phenylalanine
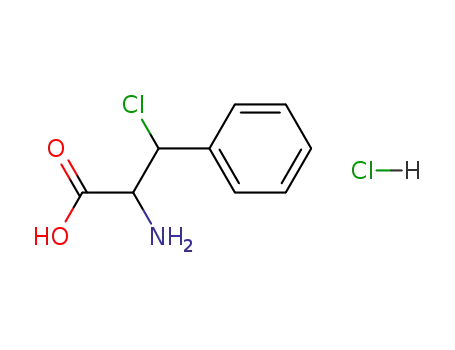
DL-β-chloro-β-phenylalanine hydrochloride
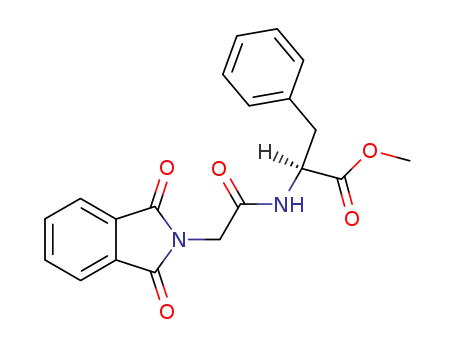
2?(1,3?dioxoisoindolin?2?yl)acetyl methyl phenylalaninate
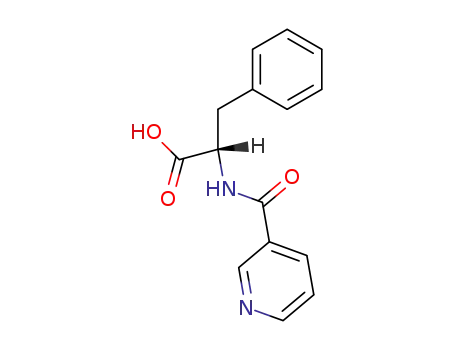
(S)-2-(nicotinamido)-3-phenylpropanoic acid
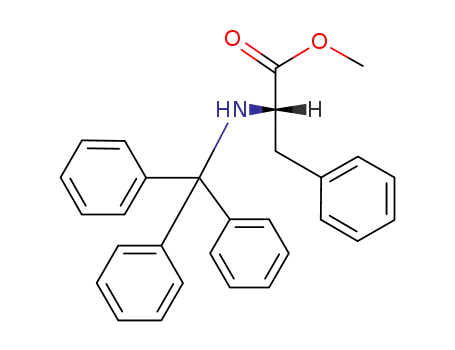
methyl N-trityl-(S)-phenylalaninate
N-benzyloxycarbonylglycyl-L-phenylalanine methyl ester
CAS:138-15-8
CAS:86028-91-3
CAS:2462-32-0
CAS:7517-19-3