Your Location:Home >Products >Biochemical Engineering >7517-19-3
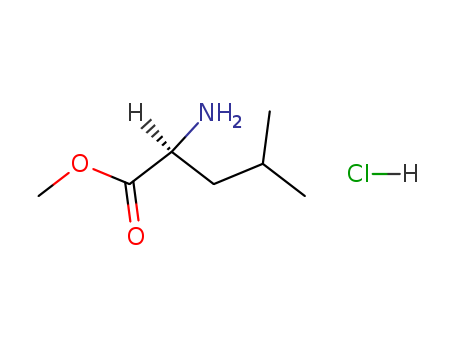

Product Details
|
Chemical Properties |
Crystalline |
|
Uses |
L-Leucine methyl ester is a protected form of L-Leucine (L330110). L-Leucine is an essential amino acid that induces a sharp decrease in blood glucose levels in individuals with idiopathic familial hypoglycemia, but has no known effects on normal, healthy individuals. L-Leucine also acts as an Isoleucine (I820210) antagonist in the rat, causing delays in growth, and is a potential tumour promoter of bladder cancer. |
InChI:InChI=1/C7H15NO2.ClH/c1-5(2)4-6(8)7(9)10-3;/h5-6H,4,8H2,1-3H3;1H
Analysis of artworks and identification ...
Ten chiral methyl 2-(2-oxo-2H-benzo[e][1...
Two new series of hitherto unknown dipep...
We describe the development and use of c...
A series of sulfur- and selenium-bearing...
methanol

(R)-leucine

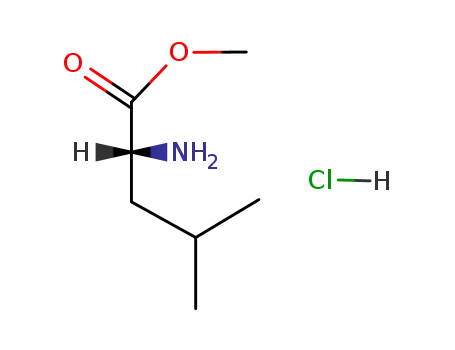
(R)-leucine methyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
With
sulfuryl dichloride;
at 0 - 20 ℃;
for 16h;
Inert atmosphere;
|
100% |
|
With
thionyl chloride;
at 20 ℃;
|
99% |
|
With
thionyl chloride;
for 12h;
Ambient temperature;
|
94% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 4.5h;
|
92% |
|
With
acetyl chloride;
for 18h;
|
91% |
|
With
thionyl chloride;
at 40 ℃;
for 3h;
|
86% |
|
With
thionyl chloride;
for 1.5h;
Heating;
|
85% |
|
With
thionyl chloride;
at 0 - 22 ℃;
for 26h;
|
85% |
|
With
hydrogenchloride;
at 20 ℃;
for 24h;
|
84% |
|
With
thionyl chloride;
Ambient temperature;
|
|
|
With
thionyl chloride;
at 40 ℃;
|
|
|
methanol;
With
thionyl chloride;
at 0 ℃;
for 0.5h;
(R)-leucine;
Heating;
|
|
|
With
thionyl chloride;
|
|
|
With
acetyl chloride;
for 2h;
Heating;
|
|
|
With
thionyl chloride;
for 3h;
Reflux;
|
|
|
With
thionyl chloride;
|
|
|
methanol; (R)-leucine;
With
thionyl chloride;
for 6h;
Reflux;
With
hydrogenchloride;
In
water;
|
|
|
methanol;
With
thionyl chloride;
at -5 - 5 ℃;
for 1.516h;
(R)-leucine;
at 30 - 45 ℃;
for 8h;
|
|
|
With
thionyl chloride;
at 0 - 20 ℃;
|
|
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
Reflux;
|
|
|
With
thionyl chloride;
at 0 ℃;
Reflux;
|
6.08 g |
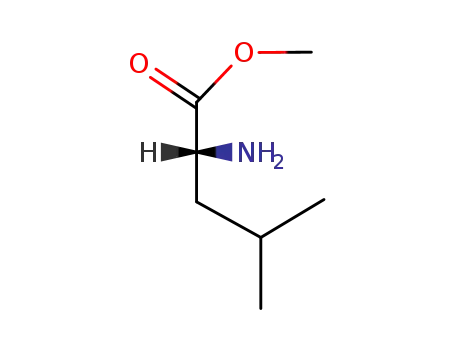
D-leucine methyl ester


(R)-leucine methyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
methanol;
|
70% |

methanol
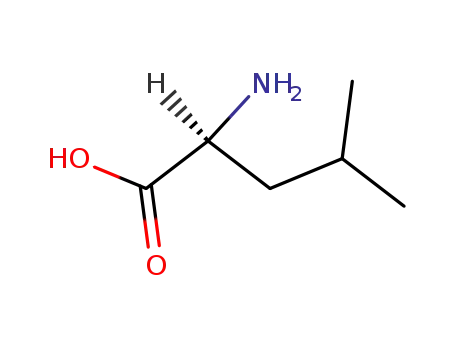
L-leucine
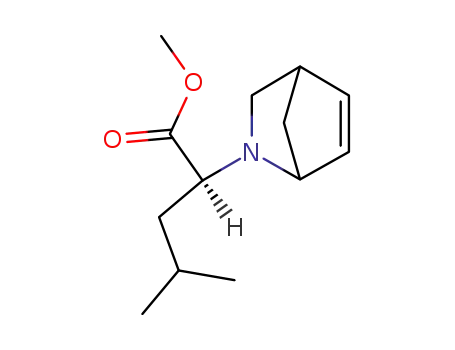
(S)-2-(2-Aza-bicyclo[2.2.1]hept-5-en-2-yl)-4-methyl-pentanoic acid methyl ester
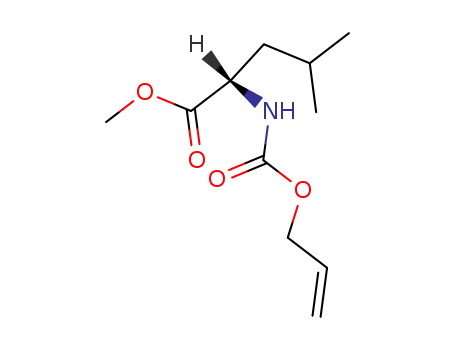
Alloc-Leu-OMe
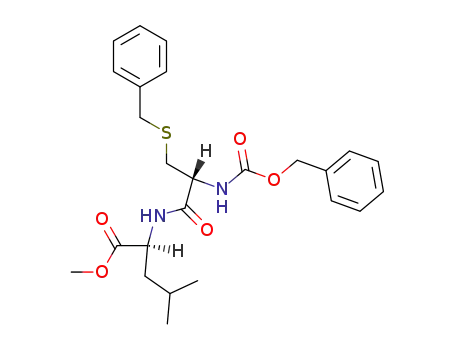
Carbobenzoxy-(S-benzyl-Cys)-Leu-methylester
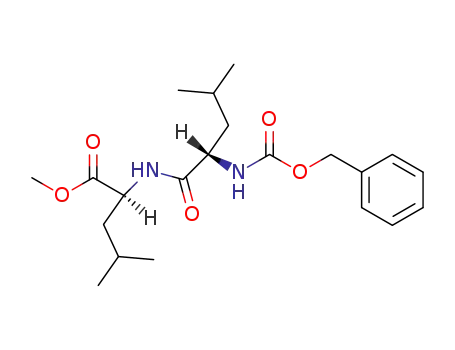
Cbz-L-Leu-L-Leu-OMe
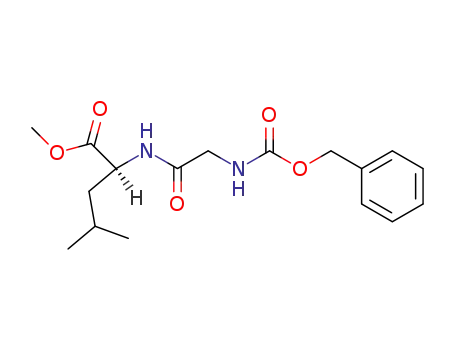
N-benzyloxycarbonylglycyl-L-leucine methyl ester
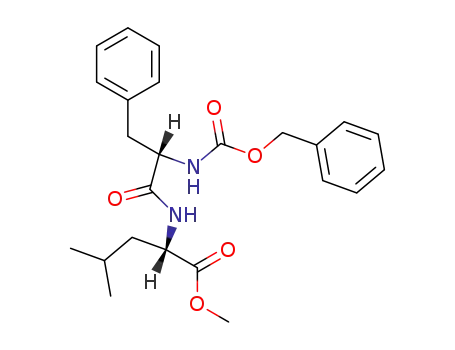
Cbz-L-Phe-L-Leu-OMe
CAS:138-15-8
CAS:86028-91-3
CAS:7524-50-7
CAS:87745-27-5