Your Location:Home >Products >Biochemical Engineering >82689-19-8
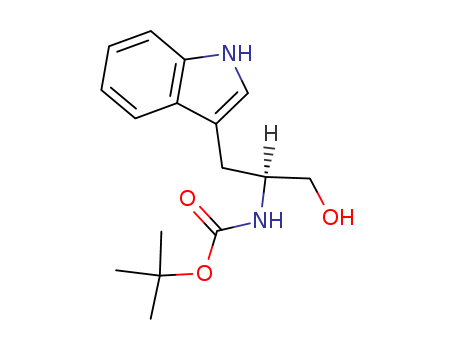

Product Details
|
Chemical Properties |
White to off-white powder |
InChI:InChI=1/C16H22N2O3/c1-16(2,3)21-15(20)18-12(10-19)8-11-9-17-14-7-5-4-6-13(11)14/h4-7,9,12,17,19H,8,10H2,1-3H3,(H,18,20)/t12-/m0/s1
The invention provides a preparation met...
A general strategy for asymmetric approa...
Drug conjugates having formula [D-(X) b ...
In a focused exploration, we designed, s...
![methyl (2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(1H-indol-3-yl)propanoate](/upload/2023/6/daf83eb9-4145-41a1-b76e-422b257e4527.png)
methyl (2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(1H-indol-3-yl)propanoate

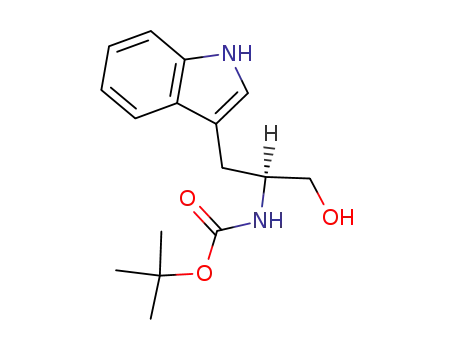
N-t-butoxycarbonyl-tryptophanol
| Conditions | Yield |
|---|---|
|
methyl (2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(1H-indol-3-yl)propanoate;
With
sodium tetrahydroborate;
In
ethanol;
at 0 - 30 ℃;
for 10h;
With
ammonium chloride;
In
ethanol;
at 0 - 5 ℃;
|
95% |
|
With
sodium tetrahydroborate; lithium chloride;
In
tetrahydrofuran;
at 20 ℃;
Inert atmosphere;
|
93% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
for 4h;
|
86% |
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
|
86% |
|
With
sodium tetrahydroborate; lithium chloride;
In
tetrahydrofuran; ethanol;
|
|
|
With
methanol; lithium borohydride;
In
tetrahydrofuran;
at 20 ℃;
for 1.5h;
Inert atmosphere;
|
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 - 25 ℃;
for 4h;
|
|
|
With
lithium aluminium tetrahydride;
In
tetrahydrofuran;
at 0 - 25 ℃;
for 4h;
|
|
|
With
sodium tetrahydroborate;
|
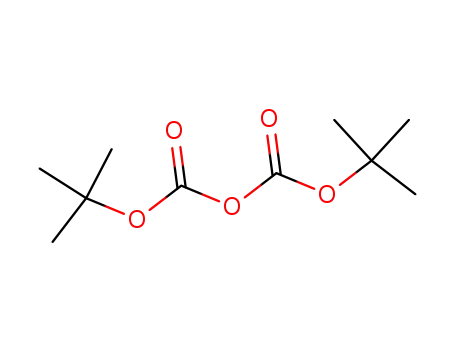
di-tert-butyl dicarbonate

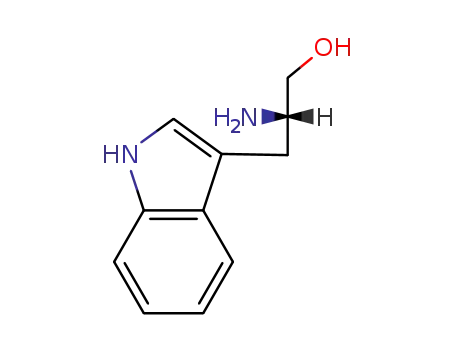
L-tryptophanol


N-t-butoxycarbonyl-tryptophanol
| Conditions | Yield |
|---|---|
|
In
1,4-dioxane; water;
at 20 ℃;
for 3h;
|
75% |
|
In
acetonitrile;
at 23 ℃;
for 3h;
|
73% |
|
In
1,4-dioxane; water;
at 25 ℃;
for 12h;
Inert atmosphere;
|
|
|
In
1,4-dioxane; water;
at 25 ℃;
|
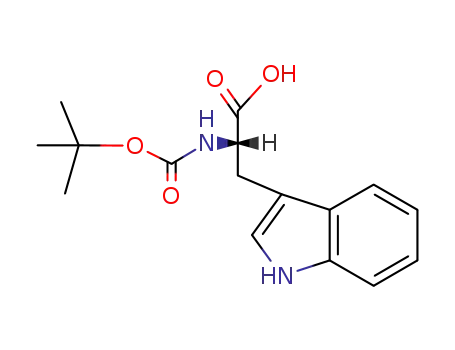
Boc-Trp-OH
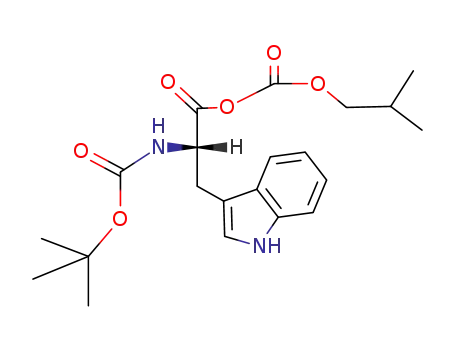
C21H28N2O6
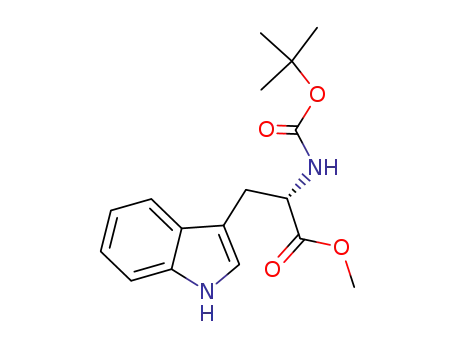
methyl (2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(1H-indol-3-yl)propanoate

di-tert-butyl dicarbonate
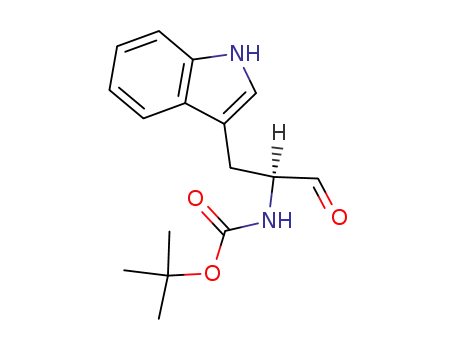
N-tert-butyloxycarbonyl-tryptophanal
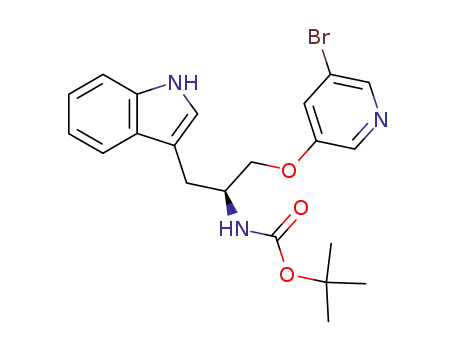
(S)-[2-(5-bromopyridin-3-yloxy)-1-(1H-indol-3-ylmethyl)ethyl]carbamic acid tert-butyl ester
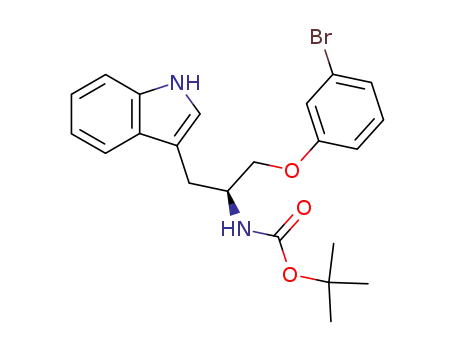
[1-(3-bromo-phenoxymethyl)-2-(1H-indol-3-yl)-ethyl]-carbamic acid tert-butyl ester
CAS:56-12-2
CAS:7531-52-4
CAS:31687-58-8
CAS:955379-18-7