Your Location:Home >Products >Biochemical Engineering >31687-58-8

Product Details
|
Chemical Properties |
White powder |
InChI:InChI=1/C16H25N3O6.C12H23N/c1-15(2,3)24-13(22)18-11(12(20)21)7-10-8-19(9-17-10)14(23)25-16(4,5)6;1-3-7-11(8-4-1)13-12-9-5-2-6-10-12/h8-9,11H,7H2,1-6H3,(H,18,22)(H,20,21);11-13H,1-10H2/t11-;/m0./s1
The tetradecapeptide Ac-Asp1-Arg2-Val3-T...
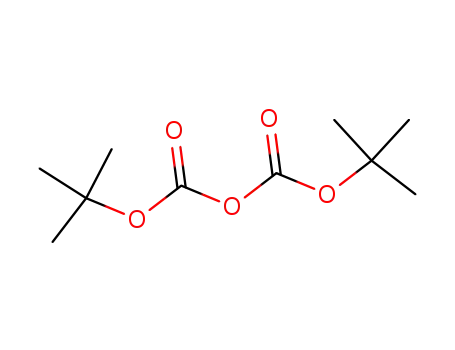
di-tert-butyl dicarbonate

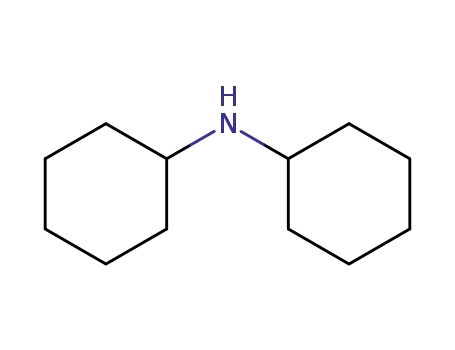
N-cyclohexyl-cyclohexanamine

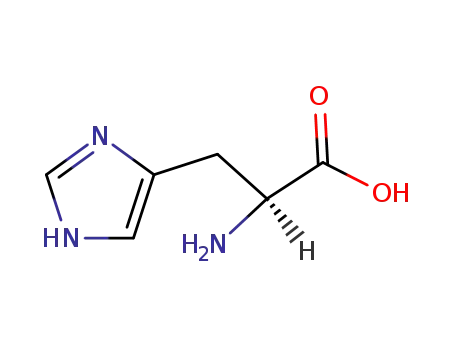
L-histidine

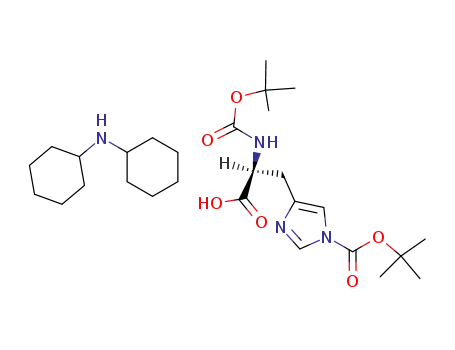
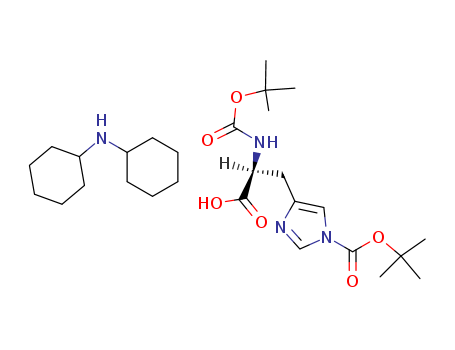
Boc-His(Boc)-OH dicyclohexylamine salt
| Conditions | Yield |
|---|---|
|
With
triethylamine;
1) water, dioxane, 30 to 45 deg C; 2) diethyl ether;
|
75% |
![N-[2-N-acetylamino-4-(3-aminophenyl)thiazole]propargylglycinamide](/upload/2023/6/86dbf158-7ace-4a7c-be44-77eca53e24df.png)
N-[2-N-acetylamino-4-(3-aminophenyl)thiazole]propargylglycinamide


Boc-His(Boc)-OH dicyclohexylamine salt

N-[2-N-acetylamino-4-(3-aminophenyl)thiazole]-[Nα,Nimidazole(di-tert-butoxycarbonyl)]histidylglycinamide
| Conditions | Yield |
|---|---|
|
With
O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine;
In
dichloromethane;
at 20 ℃;
|
94% |

di-tert-butyl dicarbonate

N-cyclohexyl-cyclohexanamine

L-histidine
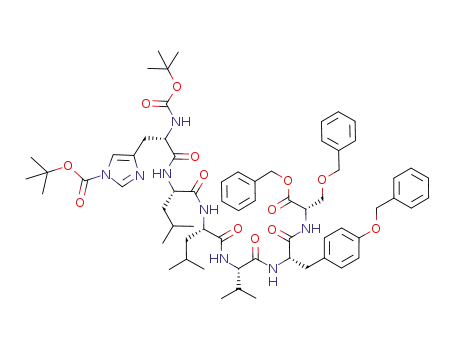
Nα-t-butoxycarbonyl-Nim-t-butoxycarbonylhistidyl-leucyl-leucylvalyl-O-benzyltyrosyl-O-benzylserine benzyl ester
CAS:138-15-8
CAS:56265-06-6
CAS:108607-02-9
CAS:82689-19-8