Your Location:Home >Products >Biochemical Engineering >17342-08-4
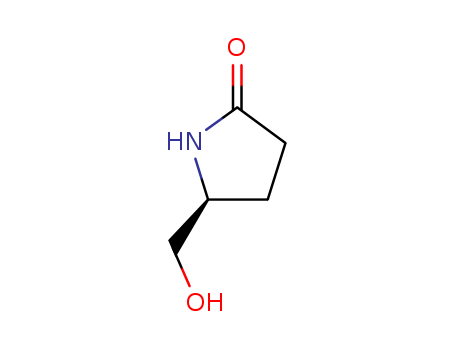

Product Details
|
Chemical Properties |
white to light yellow crystals or |
|
Uses |
(S)-Pyroglutaminol (cas# 17342-08-4) is a compound useful in organic synthesis. |
The disclosure provides at least one ent...
In this work, we provide a brief overvie...
The invention discloses a biphenyl compo...
A library of functionalized chiral pyrro...
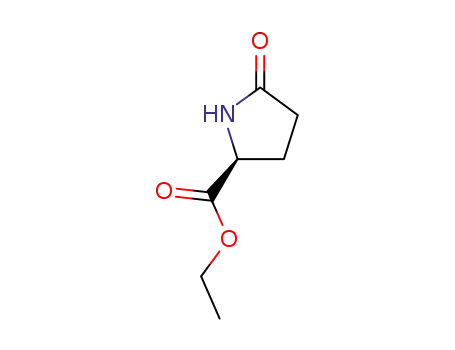
ethyl (S)-pyroglutamate

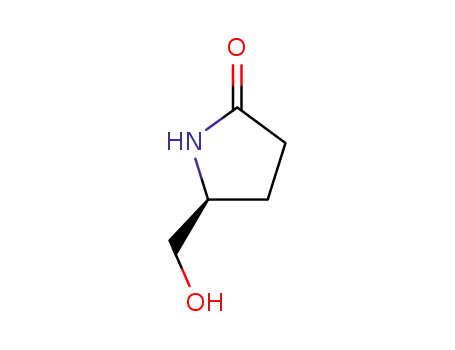
(S)-Pyroglutaminol
| Conditions | Yield |
|---|---|
|
With
potassium borohydride; lithium chloride;
In
tetrahydrofuran;
at -15 - 20 ℃;
for 2h;
Reagent/catalyst;
|
95% |
|
With
lithium borohydride;
In
tetrahydrofuran;
at 20 ℃;
for 48h;
|
90% |
|
With
sodium tetrahydroborate;
In
ethanol;
|
83% |
|
With
sodium tetrahydroborate; ethanol;
at 0 - 20 ℃;
for 6h;
|
83% |
|
With
sodium tetrahydroborate;
In
tetrahydrofuran; methanol;
at 5 ℃;
for 1h;
|
80% |
|
With
lithium borohydride;
In
tetrahydrofuran;
for 16h;
Ambient temperature;
|
78% |
|
With
sodium tetrahydroborate; ethanol;
at 0 - 5 ℃;
|
78% |
|
With
sodium tetrahydroborate; lithium chloride;
In
tetrahydrofuran; ethanol;
at 20 ℃;
for 15h;
|
73% |
|
With
sodium tetrahydroborate; lithium chloride;
Yield given. Multistep reaction;
1) diglyme, THF, 20 min.; 2) 9h, room temp., THF;
|
|
|
With
lithium borohydride;
In
tetrahydrofuran; dichloromethane;
for 2h;
Ambient temperature;
|
|
|
With
sodium tetrahydroborate;
In
water;
for 1.5h;
Ambient temperature;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
at 0 - 20 ℃;
for 1.5h;
|
31.2 g |
|
ethyl (S)-pyroglutamate;
With
sodium tetrahydroborate;
In
water;
at 20 ℃;
In
water; acetone;
at 0 ℃;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
at 20 ℃;
for 22h;
|
|
|
With
sodium tetrahydroborate; ethanol; lithium chloride;
In
tetrahydrofuran;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
at 20 ℃;
for 48h;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
|
|
|
ethyl (S)-pyroglutamate;
With
sodium tetrahydroborate;
In
ethanol;
at 10 - 12 ℃;
for 17h;
With
hydrogenchloride;
In
ethanol; water;
pH=~ 3;
|
|
|
ethyl (S)-pyroglutamate;
With
sodium tetrahydroborate;
In
ethanol;
at 0 - 20 ℃;
With
acetic acid;
In
ethanol;
at 0 - 20 ℃;
for 0.5h;
|
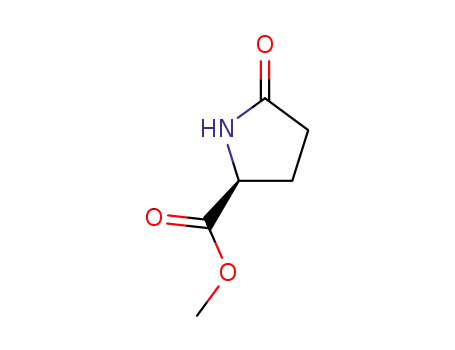
methyl (S)-pyroglutamate


(S)-Pyroglutaminol
| Conditions | Yield |
|---|---|
|
With
sodium tetrahydroborate;
In
tetrahydrofuran; methanol;
at 5 ℃;
for 1h;
|
99% |
|
With
sodium tetrahydroborate;
In
ethanol;
|
99% |
|
With
sodium tetrahydroborate;
In
ethanol;
|
99% |
|
With
sodium tetrahydroborate; ethanol;
|
99% |
|
With
sodium tetrahydroborate; isopropyl alcohol;
at 20 ℃;
for 20h;
|
98% |
|
With
sodium tetrahydroborate;
In
ethanol;
|
94% |
|
With
sodium tetrahydroborate;
In
ethanol;
|
93% |
|
With
sodium tetrahydroborate;
In
ethanol;
at 0 - 20 ℃;
|
91% |
|
With
sodium tetrahydroborate;
In
isopropyl alcohol;
at 20 ℃;
for 20h;
|
87% |
|
With
sodium tetrahydroborate;
In
isopropyl alcohol;
at 20 ℃;
for 20h;
|
86% |
|
With
sodium tetrahydroborate;
In
ethanol;
for 2h;
Ambient temperature;
|
85% |
|
With
sodium tetrahydroborate; ethanol;
at 0 - 20 ℃;
for 24h;
|
84% |
|
With
sodium tetrahydroborate;
In
ethanol;
at 20 ℃;
for 3h;
|
82.9% |
|
With
sodium tetrahydroborate;
In
ethanol;
at 20 ℃;
for 3h;
|
81% |
|
With
sodium tetrahydroborate;
In
ethanol;
at 0 - 20 ℃;
Inert atmosphere;
|
80% |
|
With
lithium borohydride;
In
methanol;
Ambient temperature;
|
80% |
|
With
sodium tetrahydroborate;
In
ethanol;
for 2h;
Ambient temperature;
|
78% |
|
With
sodium tetrahydroborate;
In
ethanol;
for 2h;
Ambient temperature;
|
75% |
|
With
sodium tetrahydroborate;
In
ethanol;
|
71% |
|
With
sodium tetrahydroborate; ethanol;
at 20 ℃;
for 2h;
|
70% |
|
With
sodium tetrahydroborate;
In
ethanol;
for 4h;
Ambient temperature;
|
60% |
|
With
sodium tetrahydroborate;
In
ethanol;
at 20 ℃;
for 2h;
Inert atmosphere;
Cooling with ice;
|
59% |
|
With
sodium tetrahydroborate; ethanol;
at 20 ℃;
Cooling with ice;
|
59% |
|
With
sodium tetrahydroborate;
In
ethanol;
at 0 - 20 ℃;
Inert atmosphere;
|
53% |
|
|
|
|
With
sodium tetrahydroborate;
Yield given;
|
|
|
With
sodium tetrahydroborate;
|
|
|
With
sodium tetrahydroborate; ethanol;
at 0 ℃;
for 12h;
|
|
|
With
sodium tetrahydroborate;
In
tetrahydrofuran; methanol;
for 1h;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
at 0 - 20 ℃;
for 18h;
|
11.5 g |
|
With
lithium borohydride;
In
tetrahydrofuran;
at 10 - 40 ℃;
for 5h;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
at 20 ℃;
|
3.3 g |
|
With
sodium tetrahydroborate;
In
ethanol;
at 20 ℃;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
at 20 ℃;
Cooling with ice;
Inert atmosphere;
|
|
|
With
sodium tetrahydroborate;
In
ethanol;
at 0 - 20 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
|
15 g |
|
With
sodium tetrahydroborate;
In
tetrahydrofuran; methanol;
for 8h;
Cooling with ice;
|
3.8 g |
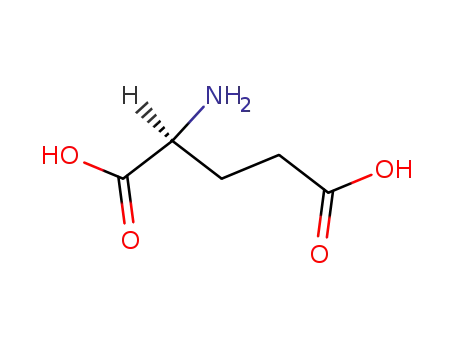
L-glutamic acid

ethyl (S)-pyroglutamate

methyl (S)-pyroglutamate
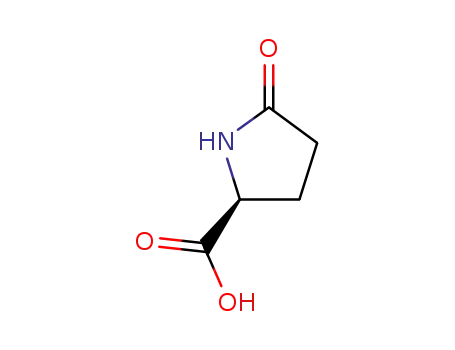
L-Pyroglutamic acid
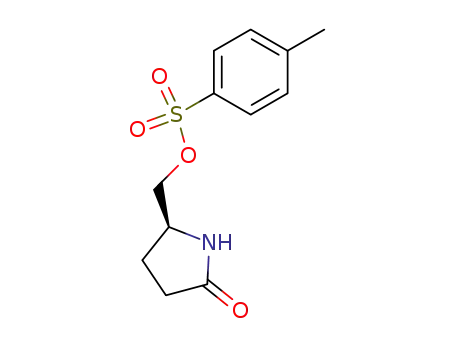
(S)-toluene-4-sulfonic acid 5-oxo-pyrrolidin-2-ylmethyl ester
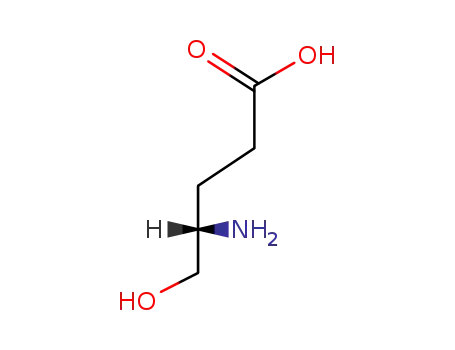
(S)-4-Amino-5-hydroxypentanoic Acid
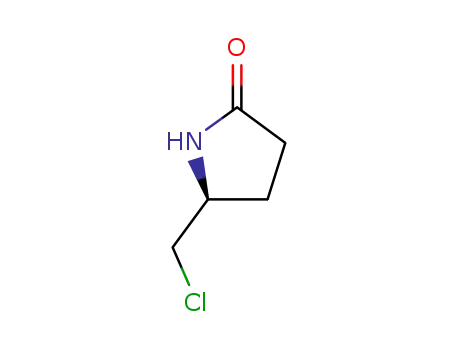
(-)-(5S)-5-(Chloromethyl)-2-pyrrolidinone
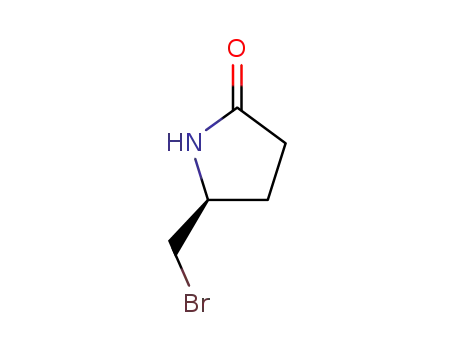
(S)-(-)-5-(Bromomethyl)-2-pyrrolidinone
CAS:56-12-2
CAS:7531-52-4
CAS:50890-96-5
CAS:100047-42-5