Your Location:Home >Products >100047-42-5

Product Details
The present invention relates to a New M...
The present invention provides compounds...
The invention provides novel compounds h...
The invention relates to compounds of Fo...
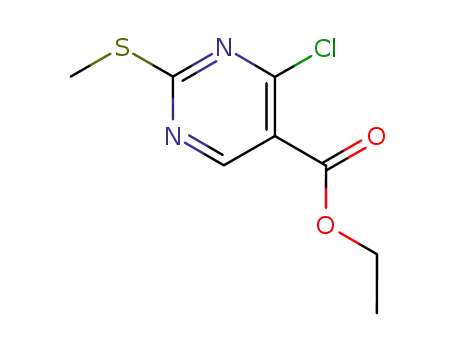
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester

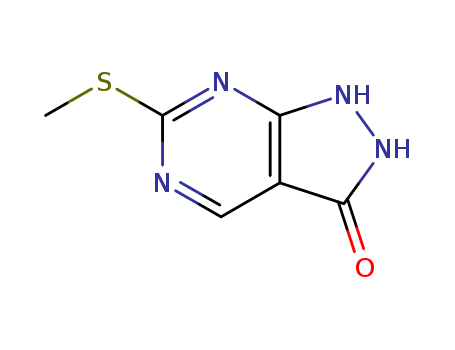
![6-(methylsulfanyl)-1H,2H,3H-pyrazolo[3,4-d]pyrimidin-3-one](/upload/2023/6/8964ec38-98a9-4f81-ac13-23091a0f4aa4.png)
6-(methylsulfanyl)-1H,2H,3H-pyrazolo[3,4-d]pyrimidin-3-one
| Conditions | Yield |
|---|---|
|
With
hydrazine;
at 20 ℃;
for 0.0833333h;
Microwave irradiation;
|
97.1% |
|
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester;
With
hydrazine;
In
ethanol;
With
sodium hydroxide;
|
85% |
|
4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester;
With
hydrazine hydrate;
In
ethanol;
at 20 ℃;
for 1h;
With
water; sodium hydroxide;
at 100 ℃;
for 0.333333h;
|
|
|
Multi-step reaction with 2 steps
1: hydrazine hydrate / ethanol / 1 h / 0 - 25 °C / Inert atmosphere
2: potassium hydroxide; acetic acid / water / 0.25 h / 0 °C / Reflux; Inert atmosphere
With
hydrazine hydrate; acetic acid; potassium hydroxide;
In
ethanol; water;
|
|
|
Multi-step reaction with 2 steps
1: hydrazine hydrate / ethanol / 1 h / 0 °C
2: water; potassium hydroxide / 1 h / 100 °C
With
water; hydrazine hydrate; potassium hydroxide;
In
ethanol;
|
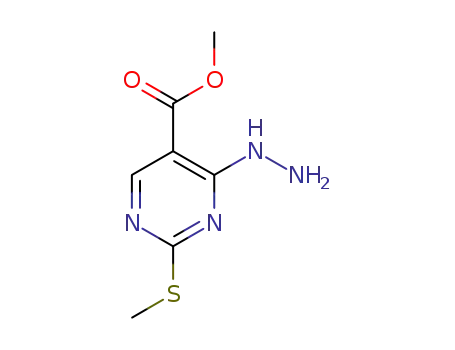
4-hydrazino-2-methylsulfanylpyrimidine-5-carboxylic acid methyl ester

![6-(methylsulfanyl)-1H,2H,3H-pyrazolo[3,4-d]pyrimidin-3-one](/upload/2023/6/8964ec38-98a9-4f81-ac13-23091a0f4aa4.png)
6-(methylsulfanyl)-1H,2H,3H-pyrazolo[3,4-d]pyrimidin-3-one
| Conditions | Yield |
|---|---|
|
With
acetic acid; potassium hydroxide;
In
water;
at 0 ℃;
for 0.25h;
Reflux;
Inert atmosphere;
|
66% |

4-chloro-2-methanesulfanylpyrimidine-5-carboxylic acid ethyl ester
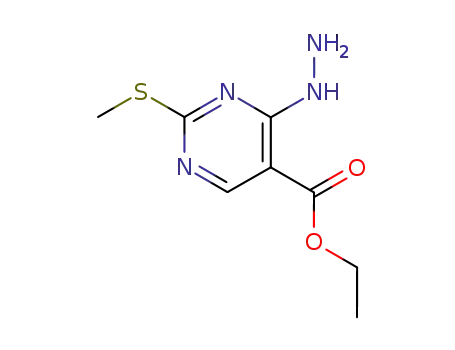
ethyl 4-hydrazinyl-2-(methylsulfanyl)pyrimidine-5-carboxylate
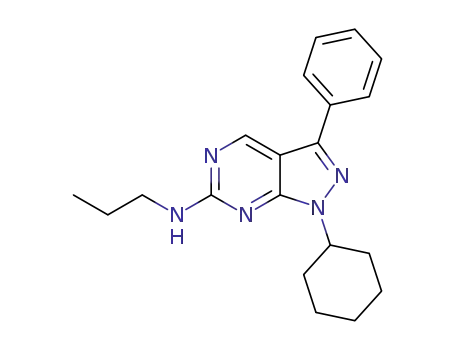
1-cyclohexyl-3-phenyl-N-propyl-1H-pyrazolo[3,4-d]pyrimidin-6-amine
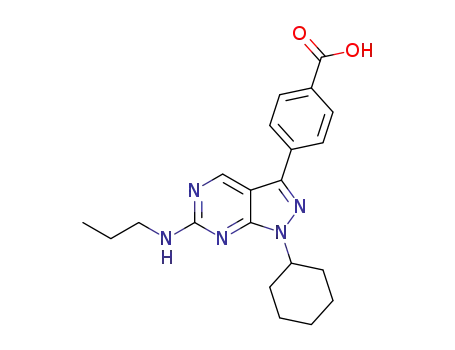
C21H25N5O2
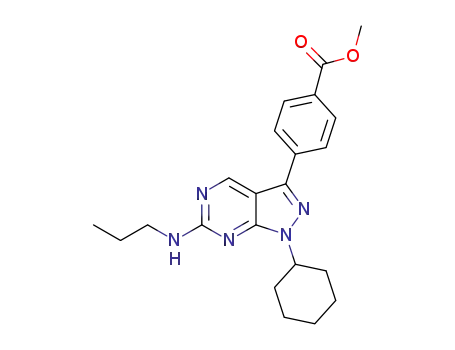
C22H27N5O2
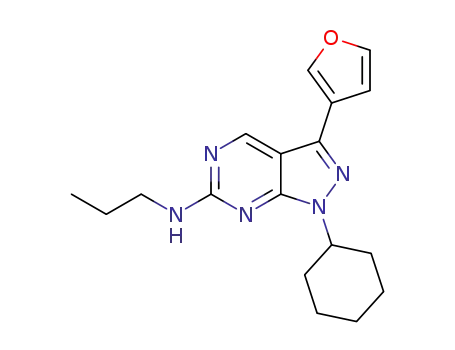
C18H23N5O
CAS:86028-91-3
CAS:34592-47-7
CAS:17342-08-4
CAS:112110-44-8