Your Location:Home >Products >112110-44-8
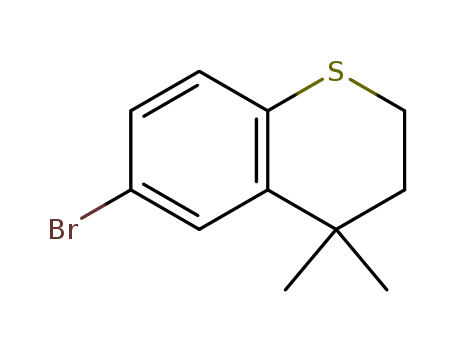

Product Details
InChI:InChI=1/C11H13BrS/c1-11(2)5-6-13-10-4-3-8(12)7-9(10)11/h3-4,7H,5-6H2,1-2H3
We report a general preparation of aryla...
The addition of nucleophilic organometal...
A Br?nsted acid-catalyzed 1,4-addition h...
One-pot synthesis of 6-bromo-4,4-dimethy...
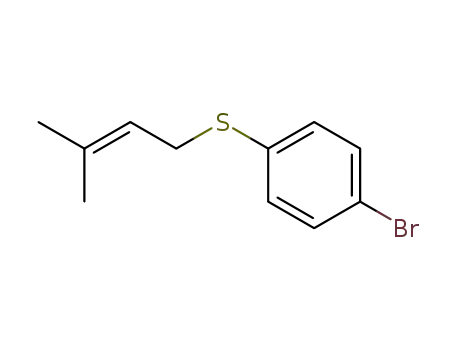
1-Bromo-4-(3-methylbut-2-enylsulphanyl)benzene

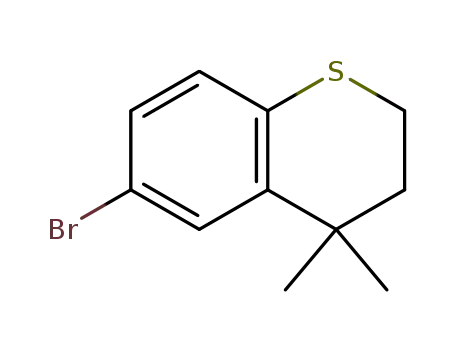
6-bromo-4,4-dimethyl-3,4-dihydro-2H-1-benzothiopyran
| Conditions | Yield |
|---|---|
|
With
polyphosphoric acid;
In
toluene;
at 100 ℃;
for 48h;
Inert atmosphere;
|
86% |
|
With
polyphosphoric acid;
In
toluene;
at 100 ℃;
for 48h;
Inert atmosphere;
Sealed tube;
|
53% |
|
With
phosphorus pentaoxide; methanesulfonic acid;
|
|
|
With
methanesulfonic acid; phosphorus pentoxide;
at 20 ℃;
for 2h;
|
|
|
With
phosphorus pentoxide;
In
methanesulfonic acid;
at 20 ℃;
for 2h;
|
![1-bromo-4-[(3-methylbut-3-en-1-yl)sulfanyl]benzene](/upload/2023/6/2765fba4-79c9-4357-a300-1964a5d03cbd.png)
1-bromo-4-[(3-methylbut-3-en-1-yl)sulfanyl]benzene


6-bromo-4,4-dimethyl-3,4-dihydro-2H-1-benzothiopyran
| Conditions | Yield |
|---|---|
|
With
aluminum (III) chloride;
In
dichloromethane;
at 0 - 20 ℃;
for 2h;
|

1-Bromo-4-(3-methylbut-2-enylsulphanyl)benzene
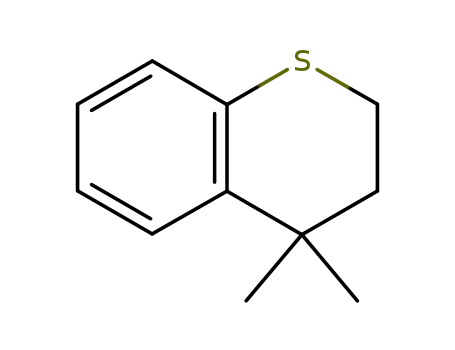
4,4-dimethyl-3,4-dihydro-2H-1-benzothiopyran
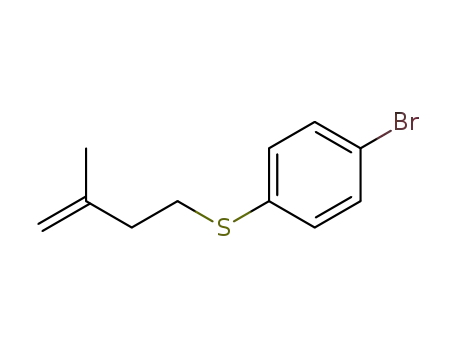
1-bromo-4-[(3-methylbut-3-en-1-yl)sulfanyl]benzene
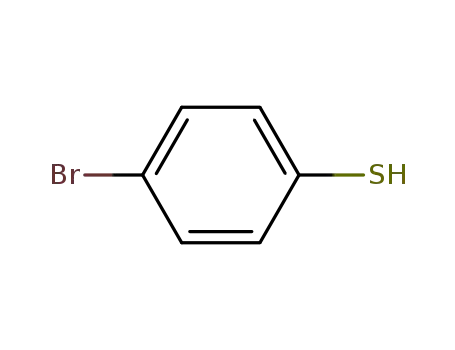
para-bromobenzenethiol
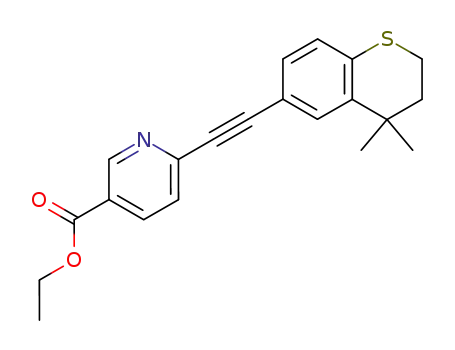
tazarotene
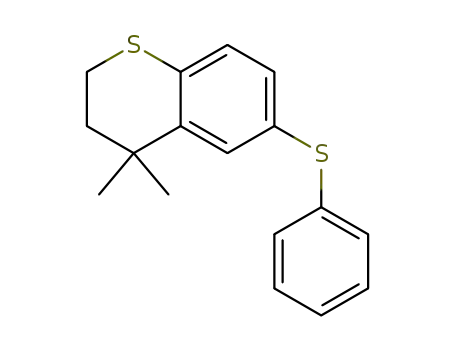
4,4-dimethyl-6-(phenylthio)thiochromane
CAS:15295-77-9
CAS:56-12-2
CAS:100047-42-5
CAS:146645-63-8