Your Location:Home >Products >Biochemical Engineering >15295-77-9
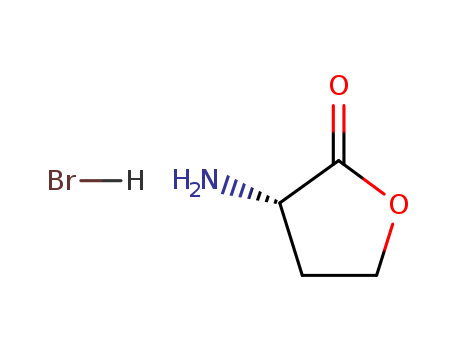

Product Details
|
Chemical Properties |
WHITE TO SLIGHTLY YELLOW CRYSTALLINE POWDER |
| Description | L-Homoserine Lactone Hydrobromide is a reactant in the synthesis of tritium labeled and photoactivatable N-acyl-L-homoserine lactones. |
|
Uses |
L-Homoserine lactone hydrobromide is used as a building block. |
InChI:InChI=1/C4H7NO2.BrH/c5-3-1-2-7-4(3)6;/h3H,1-2,5H2;1H/t3-;/m0./s1
l- or d-homoserine lactone hydrobromide was synthesized … and was then reacted with l-homoserine lactone (route A). In … reaction with l-homoserine lactone or (d-homoserine lactone for …
A homoserine lactone hydrobromide 19, was prepared by refluxing l-methionine 18 with … N-(3-oxo-octanoyl)-l-homoserine lactone (OOHL) binding site and binding ability of the …
15 g of the homoserine lactone hydrobromide (ca. 80%). … 60 mmol) of the homoserine lactone hydrobromide was sus… The hydrobromide gradually dissolved and KBr precipitated. …
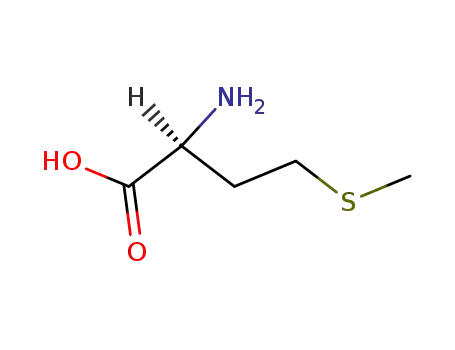
L-methionine

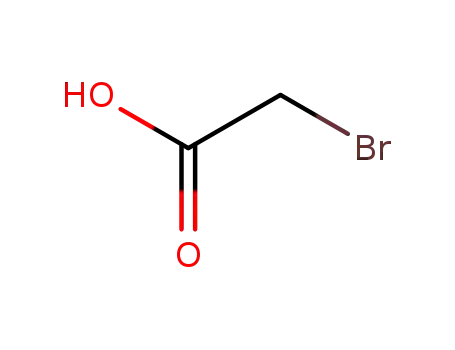
bromoacetic acid

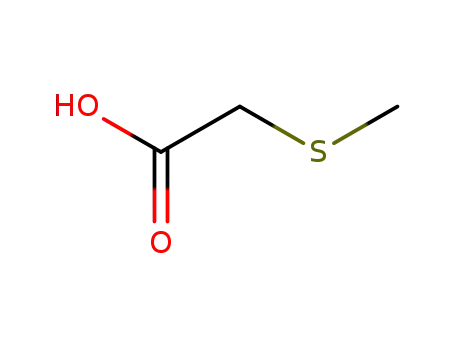
methylsulfanyl-acetic acid

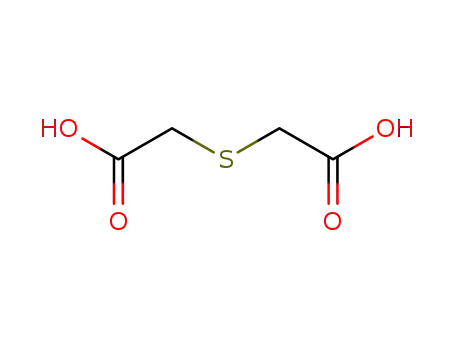
thiodiacetic acid

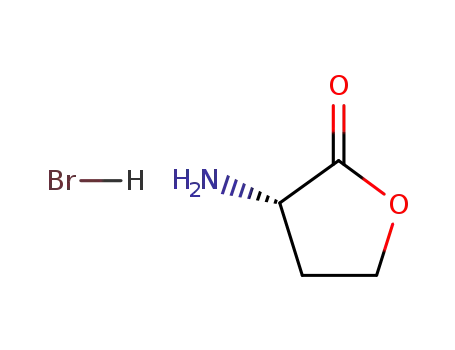
(S)-(-)-α-amino-γ-butyrolactone hydrobromide
| Conditions | Yield |
|---|---|
|
In water; at 80 ℃; for 8h; Overall yield = 303.4 g;
|

L-methionine


(S)-(-)-α-amino-γ-butyrolactone hydrobromide
| Conditions | Yield |
|---|---|
|
With bromoacetic acid; In water; acetic acid; isopropyl alcohol; Reflux; Inert atmosphere;
|
86% |
|
With bromoacetic acid; In water; acetic acid; isopropyl alcohol; for 9h; Heating;
|
81% |
|
L-methionine; With acetic acid; bromoacetic acid; In water; isopropyl alcohol; Reflux;
With hydrogenchloride; at 50 ℃; for 0.166667h;
|
75% |
|
L-methionine; With acetic acid; bromoacetic acid; In water; isopropyl alcohol; for 12h; Inert atmosphere; Reflux;
With hydrogen bromide; In isopropyl alcohol; Cooling;
|
75% |
|
L-methionine; With acetic acid; bromoacetic acid; In water; isopropyl alcohol; at 20 - 95 ℃; Industry scale;
With hydrogenchloride; In 1,4-dioxane; isopropyl alcohol; at 20 - 60 ℃; Industry scale;
|
70% |
|
L-methionine; With acetic acid; bromoacetic acid; In water; isopropyl alcohol; Reflux;
With hydrogenchloride; at 55 ℃; for 0.5h;
|
55% |
|
L-methionine; With acetic acid; bromoacetic acid; In water; isopropyl alcohol; for 2h; Reflux;
With hydrogenchloride; In 1,4-dioxane; water; at 50 ℃;
|
52% |
|
L-methionine; With acetic acid; bromoacetic acid; In water; isopropyl alcohol; for 8h; Reflux;
With hydrogenchloride; In 1,4-dioxane; at 20 - 50 ℃; for 5.16667h;
|
52% |
|
With acetic acid; bromoacetic acid; In water; isopropyl alcohol; for 2h; Reflux;
|
45% |
|
Yield given. Multistep reaction;
|
|
|
L-methionine; With bromoacetic acid; In water; acetic acid; isopropyl alcohol; for 2h; Heating / reflux;
With hydrogenchloride; In 1,4-dioxane; water; at 50 ℃;
|
|
|
With bromoacetic acid; In [D3]acetonitrile; water; isopropyl alcohol; Reflux;
|
|
|
L-methionine; With acetic acid; bromoacetic acid; In water; isopropyl alcohol; Reflux;
With hydrogen bromide; In isopropyl alcohol;
|

L-methionine

bromoacetic acid
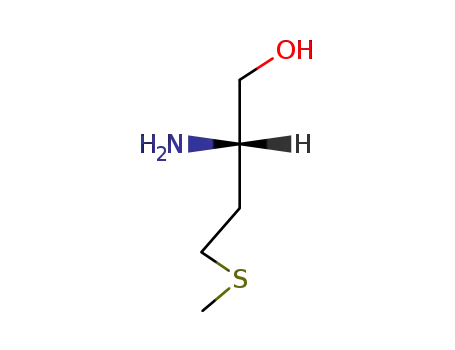
(S)-methioninol
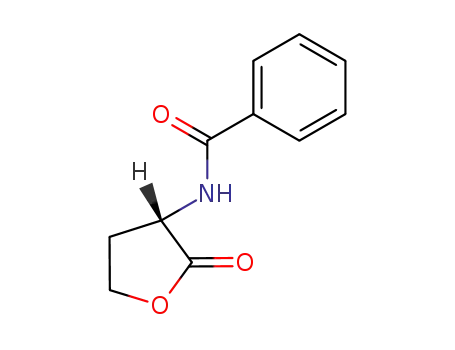
(S)-2-benzoylamino-γ-butyrolactone
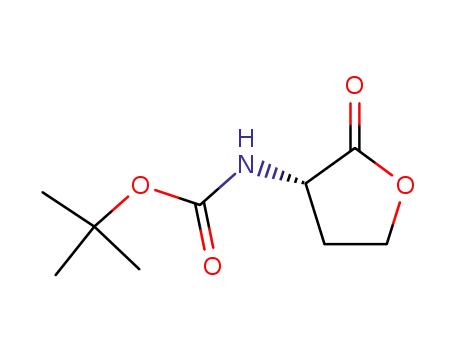
tert-butyl (3S)-2-oxotetrahydrofuran-3-ylcarbamate
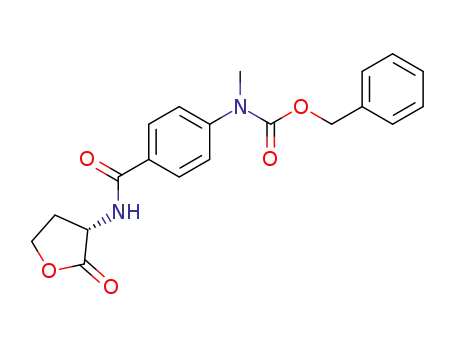
(2S)-2-N-[4-(N-benzyloxycarbonyl-N-methyl)aminobenzoyl]aminobutyrolactone
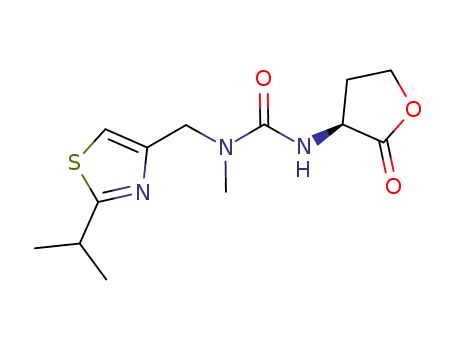
3-methyl-1-[(3S)-2-oxooxolan-3-yl]-3-{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}urea
CAS:138-15-8
CAS:56265-06-6
CAS:62965-35-9
CAS:166108-71-0