Your Location:Home >Products >Biochemical Engineering >62965-35-9

Product Details
|
Chemical Properties |
White crystalline powder |
| Description | Boc-L-tert-leucine is a amino acid additive. |
|
Uses |
Atazanavir Related Compound A. Boc-L-tert-leucine can be used as carboxylic acid additive, which provide the benzylic alcohol product in up to 42% yield, together with the ketone product in 9% yield. |
InChI:InChI=1/C11H21NO4/c1-10(2,3)7(8(13)14)12-9(15)16-11(4,5)6/h7H,1-6H3,(H,12,15)(H,13,14)/p-1/t7-/m1/s1
Pleasingly, both the reactivity and the selectivity were improved with the addition of N-Boc-L-tert-Leucine (Boc-L-Tle-OH). 3aa was obtained in high yield and excellent ee (Table 1, entry …
To a stirred solution of boc-L-tert-leucine 9 (347 mg, 1.50 mmol) and methyl(1R,2S,5S)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate hydrochloride 5 (205 mg, 1.0 mmol) in 13 …
After screening various ligands, it was found that Boc-l-tert-leucine led to optimal results, forming axially chiral products in up to 99% yield and 99% ee. …
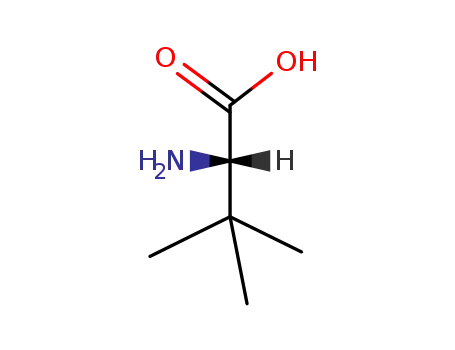
L-tert-Leucine

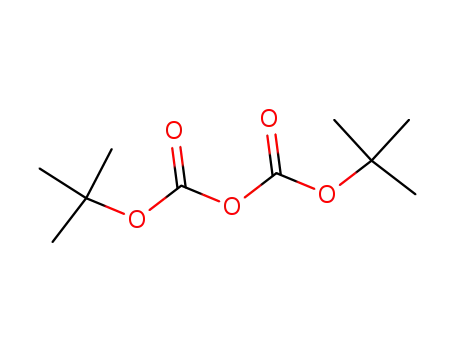
di-tert-butyl dicarbonate

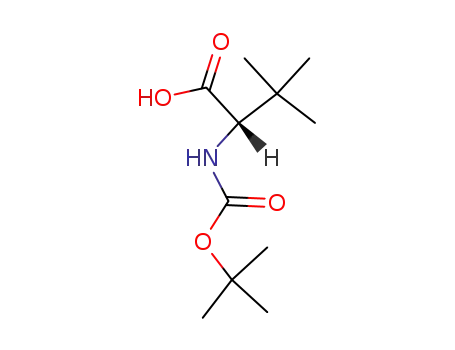
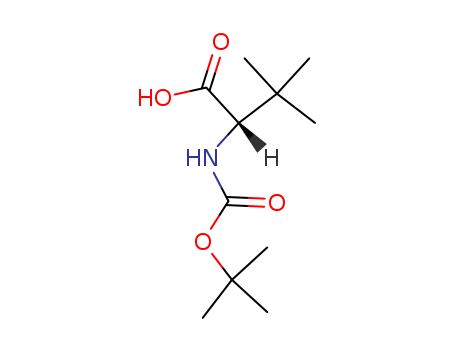
N-tert-butyloxycarbonyl-L-tert-leucine
| Conditions | Yield |
|---|---|
|
With triethylamine; In methanol; at 0 - 5 ℃;
|
100% |
|
With triethylamine; In methanol; at 0 - 5 ℃;
|
100% |
|
With sodium hydroxide; In 1,4-dioxane; at 20 ℃; for 12h;
|
100% |
|
With triethylamine; In 1,4-dioxane; water;
|
99% |
|
With sodium hydroxide; In water; tert-butyl alcohol; at 20 ℃;
|
99% |
|
With sodium hydroxide; In water; isopropyl alcohol; at 25 ℃; for 3h; pH=8.5 - 9.5; Product distribution / selectivity;
|
99% |
|
With sodium hydroxide; In water; at 7 - 20 ℃; for 14h; pH=9.4 - 10.8;
|
96% |
|
L-tert-Leucine; di-tert-butyl dicarbonate; With sodium hydroxide; In water; tert-butyl alcohol; at 20 ℃; for 18h;
With potassium hydrogensulfate; In water; pH=2;
|
95% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 20 ℃;
|
92% |
|
L-tert-Leucine; di-tert-butyl dicarbonate; With sodium hydroxide; In 1,4-dioxane; water; at 0 - 20 ℃; for 16h;
With hydrogenchloride; In water; ethyl acetate;
|
92% |
|
L-tert-Leucine; di-tert-butyl dicarbonate; With sodium hydroxide; In 1,2-dioxacyclohexane; water; at 0 - 20 ℃; for 4h; pH=8 - 9;
With potassium hydrogensulfate; In water; pH=3;
|
89% |
|
L-tert-Leucine; With sodium tetrahydroborate; iodine; In tetrahydrofuran;
di-tert-butyl dicarbonate; With triethylamine; In tetrahydrofuran;
|
89% |
|
With potassium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 12h; Inert atmosphere;
|
87% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 0 - 20 ℃; for 16h;
|
82% |
|
With triethylamine; In 1,4-dioxane; water; for 6h;
|
78% |
|
With triethylamine; In N,N-dimethyl-formamide; for 15h; Ambient temperature;
|
64% |
|
With dmap; In methanol;
|
|
|
With sodium hydroxide; In 1,4-dioxane; at 20 ℃;
|
|
|
With sodium hydroxide; In tetrahydrofuran; at 20 ℃; for 18h;
|
|
|
With sodium carbonate; In methanol; at 23 ℃; for 16h;
|
|
|
With sodium hydroxide; In 1,4-dioxane; at 20 ℃; for 14h;
|
6.7 g |
|
L-tert-Leucine; With sodium hydrogencarbonate; sodium hydroxide; Inert atmosphere;
di-tert-butyl dicarbonate; In 1,4-dioxane; for 16h; Inert atmosphere;
|
|
|
With sodium carbonate; In tetrahydrofuran; water; at 0 - 20 ℃;
|
|
|
With triethylamine; In tetrahydrofuran; water; at 20 ℃;
|
|
|
L-tert-Leucine; di-tert-butyl dicarbonate; With sodium carbonate; In tetrahydrofuran; water; at 0 - 20 ℃; for 12h;
With hydrogenchloride; In tetrahydrofuran; water; pH=2;
|
|
|
With triethylamine; In N,N-dimethyl-formamide;
|
|
|
With sodium hydroxide; at 0 - 20 ℃;
|
|
|
With sodium carbonate; In methanol; water; at 20 ℃; for 20h;
|
|
|
With sodium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 1h; Inert atmosphere;
|
|
|
With sodium carbonate; In tetrahydrofuran; water; at 0 - 20 ℃; for 12h;
|
|
|
With sodium carbonate; In tetrahydrofuran; water; at 0 - 20 ℃; for 12h;
|
|
|
With sodium carbonate; sodium hydroxide; In tetrahydrofuran; water;
|
|
|
With potassium carbonate; In water; isopropyl alcohol;
|
|
|
With sodium hydroxide; In tetrahydrofuran; water;
|
|
|
With sodium hydrogencarbonate; sodium hydroxide; In 1,4-dioxane; water; at 25 ℃; Cooling with ice;
|
NaHSO4·H2O


L-tert-Leucine


di-tert-butyl dicarbonate


N-tert-butyloxycarbonyl-L-tert-leucine
| Conditions | Yield |
|---|---|
|
With triethylamine; In 1,4-dioxane; water;
|
99% |
|
With triethylamine; In 1,4-dioxane; water;
|
99% |
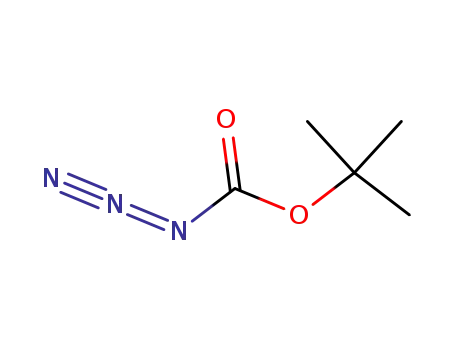
N-(tert-butyloxycarbonyl) azide

L-tert-Leucine
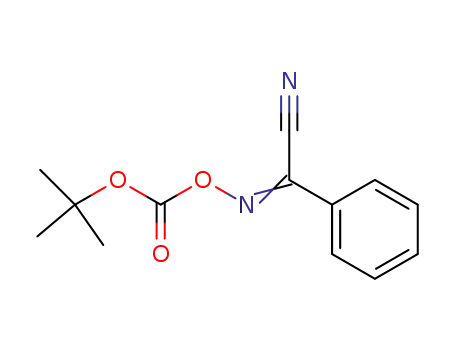
2-(tert-Butoxycarbonyloxyimino)-2-phenylacetonitrile

di-tert-butyl dicarbonate
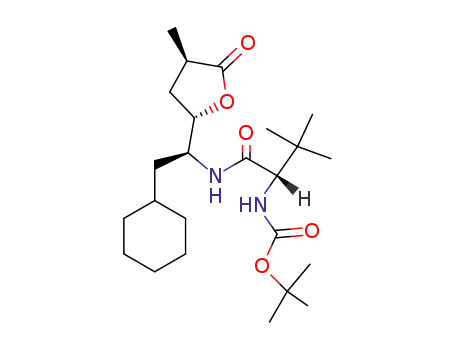
{(S)-1-[(S)-2-Cyclohexyl-1-((2S,4R)-4-methyl-5-oxo-tetrahydro-furan-2-yl)-ethylcarbamoyl]-2,2-dimethyl-propyl}-carbamic acid tert-butyl ester
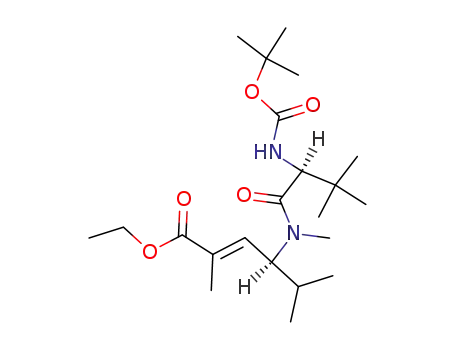
ethyl (S,E)-4-((S)-2-((tert-butoxycarbonyl)amino)-N,3,3-trimethylbutanamido)-2,5-dimethylhex-2-enoate
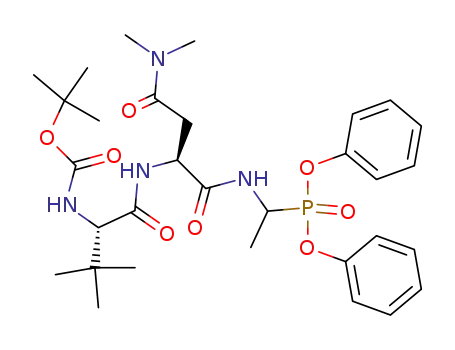
{1-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3,3-dimethyl-butyrylamino)-3-dimethylcarbamoyl-propionylamino]-ethyl}-phosphonic acid diphenyl ester
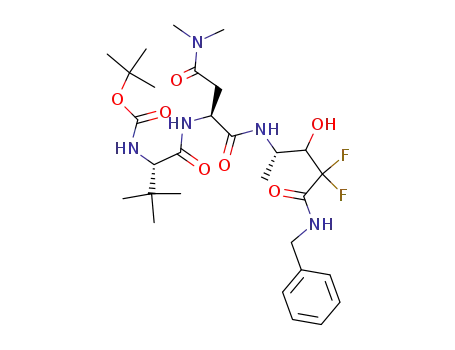
{(S)-1-[(S)-1-((S)-3-Benzylcarbamoyl-3,3-difluoro-2-hydroxy-1-methyl-propylcarbamoyl)-2-dimethylcarbamoyl-ethylcarbamoyl]-2,2-dimethyl-propyl}-carbamic acid tert-butyl ester
CAS:56-12-2
CAS:7531-52-4
CAS:13734-36-6
CAS:15295-77-9