Your Location:Home >Products >Biochemical Engineering >34592-47-7
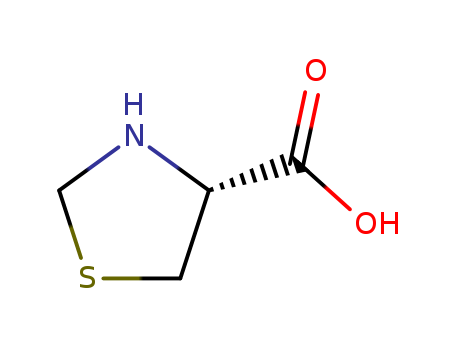

Product Details
|
Chemical Properties |
solid |
|
Uses |
L(-)-Thiazolidine-4-carboxylic acid is a proline analogue, which used in peptide coupling reactions. Thiazolidine-4-carboxylic acid (TC) is a cyclic sulfur amino acid, a condensation product of cysteine and formaldehyde. |
|
Definition |
ChEBI: An optically active version of thioproline having L-configuration. |
InChI:InChI=1/C5H7NO2S/c7-5(8)4-3(9)1-2-6-4/h4,6H,1-2H2,(H,7,8)
L-thiazolidine-4-carboxylic acid, a product of the non-enzymatic condensation of equimolar quantities of formaldehyde and L-cysteine to yield the saturated imino acid containing S as a thioether, has the ability to interfere with the utilization of proline for protein synthesis and to mimic proline in its incorporation into proteins. It can be metabolized in both bacterial and mammalian cells.
This research study explains the synthesis pertaining to certain Thiazolidine-4-carboxylic acid derivatives L2 and L1, which were synthesized in good yield via the reaction of L-Cysteine …
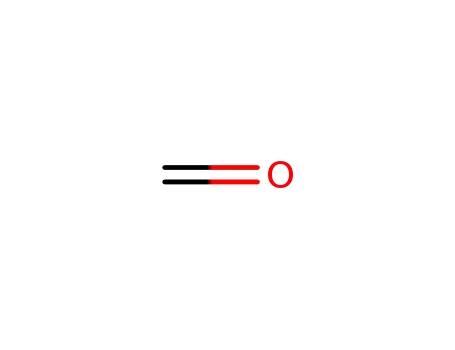
formaldehyd

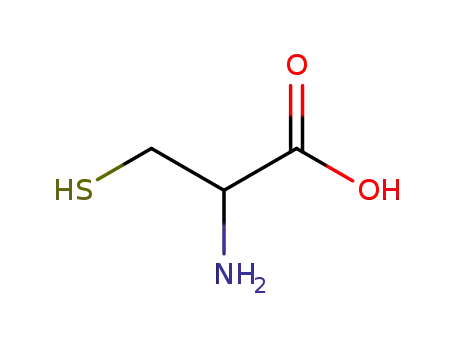
rac-cysteine

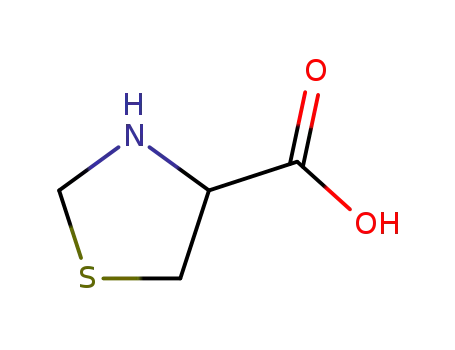
(RS)-4-thiazolidinecarboxylic acid
| Conditions | Yield |
|---|---|
|
In water; acetic acid; at 30 ℃; for 1h;
|
93.5% |
|
|
|
|
In water; for 0.25h; Concentration; Time; Heating;
|
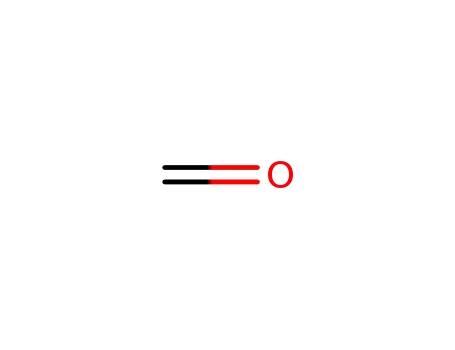
formaldehyd

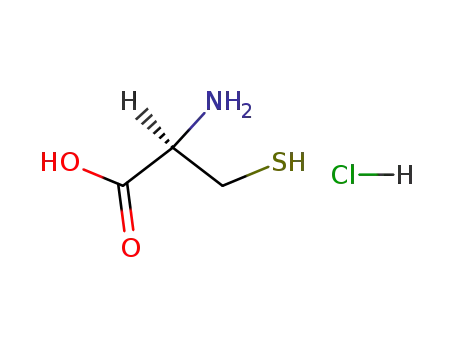
l-cysteine hydrochloride


(RS)-4-thiazolidinecarboxylic acid
| Conditions | Yield |
|---|---|
|
In water; Ambient temperature;
|
74% |
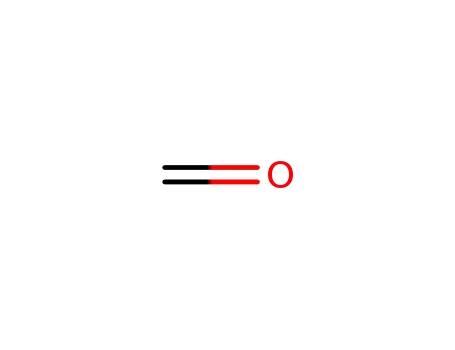
formaldehyd

l-cysteine hydrochloride

(RS)-4-thiazolidinecarboxylic acid
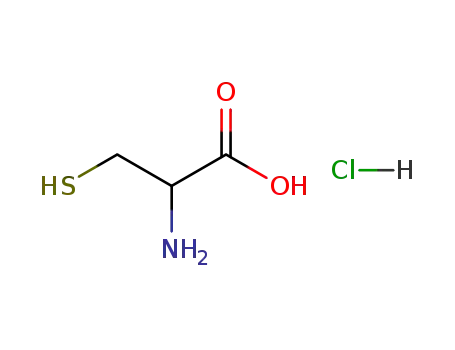
DL-cysteine hydrochloride
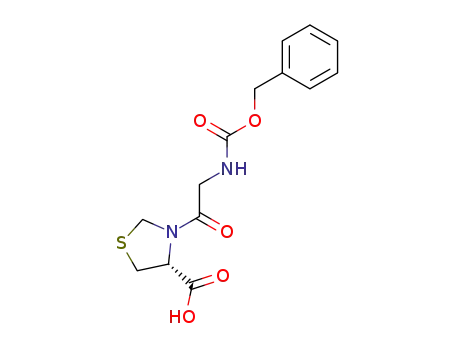
Z-Gly-P(S)
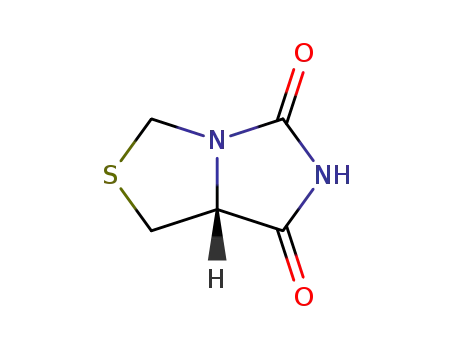
L'acide L-thiazolidinecarboxylique-4 hydantoine
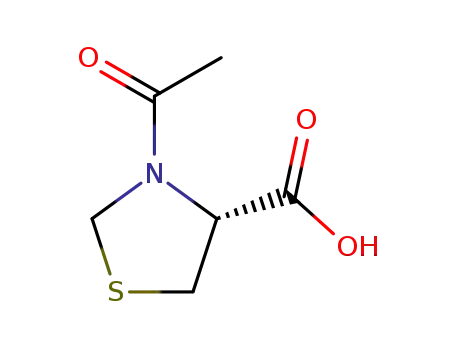
(4R)-N-acetylthiazolidine-4-carboxylic acid
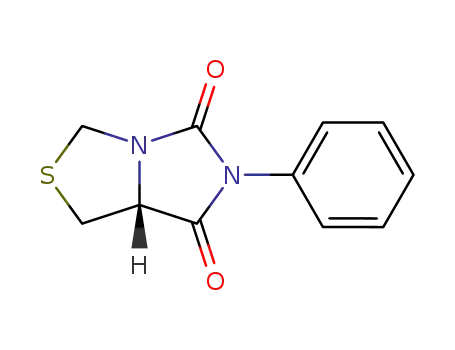
6,8-dioxo-7-phenyl-3-thia-1,7-diazabicyclo<3.3.0>octane
CAS:86028-91-3
CAS:59-51-8
CAS:56-86-0
CAS:556-50-3