Your Location:Home >Products >50890-96-5

Product Details
Herein, we report the discovery of sever...
Hepatitis C virus (HCV) is an internatio...
Novel symmetric molecules, bearing a ben...
The invention discloses compounds for in...
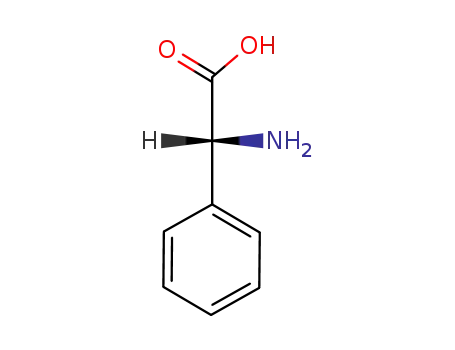
(R)-phenylglycine

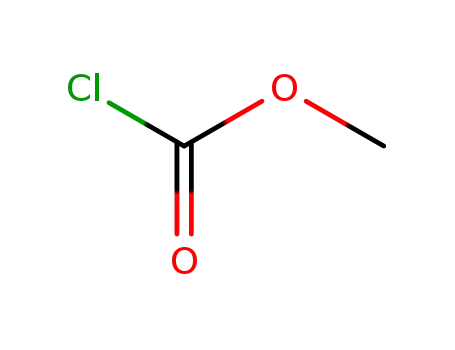
methyl chloroformate

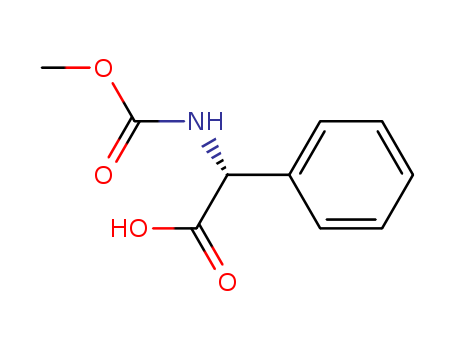
![(2R)-2-[(methoxycarbonyl)amino]-2-phenylacetic acid](/upload/2023/6/106ff3cd-1478-4165-bccb-c0ae6383f38c.png)
(2R)-2-[(methoxycarbonyl)amino]-2-phenylacetic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1.5h;
|
92% |
|
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1.33333h;
|
91% |
|
(R)-phenylglycine; methyl chloroformate;
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1h;
With
hydrogenchloride;
In
water;
|
91% |
|
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1h;
|
91% |
|
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1h;
|
91% |
|
(R)-phenylglycine; methyl chloroformate;
With
sodium hydroxide;
In
water;
at 0 - 20 ℃;
for 1.33h;
With
hydrogenchloride;
In
water;
|
91% |
|
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1.33333h;
|
91% |
|
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1.33333h;
|
91% |
|
With
sodium hydrogencarbonate;
In
tetrahydrofuran; water;
at 20 ℃;
|
80% |
|
(R)-phenylglycine;
With
sodium hydroxide;
In
water;
at 0 ℃;
for 0.0833333h;
methyl chloroformate;
In
1,4-dioxane; water;
at 25 - 30 ℃;
for 24h;
|
71% |
|
With
sodium carbonate; sodium hydroxide;
In
water;
at 20 ℃;
for 3.25h;
Cooling with ice;
|
67% |
|
With
sodium carbonate; sodium hydroxide;
In
water;
at 20 ℃;
for 3.25h;
Cooling with ice;
|
67% |
|
With
sodium carbonate; sodium hydroxide;
In
water;
at 20 ℃;
for 3.25h;
Cooling with ice;
|
67% |
|
With
sodium carbonate; sodium hydroxide;
In
water;
at 20 ℃;
for 3.25h;
Cooling with ice;
|
67% |
|
With
sodium carbonate; sodium hydroxide;
In
water;
at 20 ℃;
for 3.25h;
Cooling with ice;
|
67% |
|
With
sodium carbonate; sodium hydroxide;
In
water;
at 20 ℃;
for 3.25h;
Cooling with ice;
|
63% |
|
With
sodium hydroxide;
In
water; toluene;
at 20 ℃;
for 12h;
Cooling with ice;
|
62% |
|
(R)-phenylglycine; methyl chloroformate;
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1.33333h;
With
hydrogenchloride;
In
water;
|
|
|
(R)-phenylglycine; methyl chloroformate;
With
sodium hydroxide;
In
water;
at 0 ℃;
With
hydrogenchloride;
In
water;
|
|
|
(R)-phenylglycine;
With
lithium hydroxide;
In
water;
at 0 ℃;
methyl chloroformate;
In
water;
at 0 ℃;
for 2h;
|
|
|
(R)-phenylglycine; methyl chloroformate;
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1.33333h;
With
hydrogenchloride;
In
diethyl ether; water;
at 0 ℃;
|
|
|
(R)-phenylglycine; methyl chloroformate;
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1.33333h;
With
hydrogenchloride;
In
water;
|
|
|
(R)-phenylglycine; methyl chloroformate;
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1.33333h;
With
hydrogenchloride;
In
diethyl ether; water;
at 0 ℃;
|
|
|
With
LiOH;
In
water;
|
|
|
With
sodium hydroxide;
In
water;
at 0 ℃;
for 1.33333h;
|
|
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 0 - 20 ℃;
|
|
|
With
sodium hydroxide;
In
1,4-dioxane;
at 0 - 20 ℃;
|
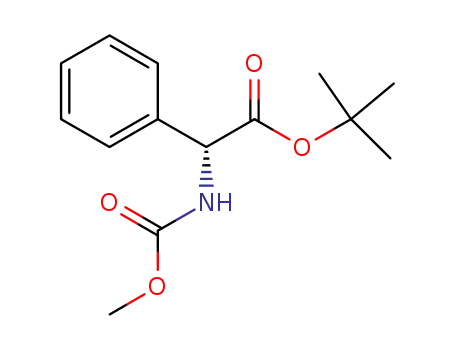
(R)-tert-butyl 2-(methoxycarbonylamino)-2-phenylacetate

![(2R)-2-[(methoxycarbonyl)amino]-2-phenylacetic acid](/upload/2023/6/106ff3cd-1478-4165-bccb-c0ae6383f38c.png)
(2R)-2-[(methoxycarbonyl)amino]-2-phenylacetic acid
| Conditions | Yield |
|---|---|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 22.1h;
Cooling with ice/water;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 22h;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
Cooling with ice;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
Cooling with ice;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 22h;
Cooling with ice;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 20h;
Cooling with ice;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 22.14h;
Cooling with ice/water;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 22h;
Cooling with ice;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 22h;
Cooling with ice;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 22.11h;
Cooling with ice;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 22h;
Cooling with ice;
|
5.57 g |
|
With
trifluoroacetic acid;
In
dichloromethane;
at 0 ℃;
for 22.1167h;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
at 0 ℃;
for 22h;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
at 0 ℃;
for 22.1167h;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
for 22.1167h;
Cooling with ice/water;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
Cooling with ice-water bath;
|
|
|
With
trifluoroacetic acid;
In
dichloromethane;
Cooling with ice;
|

(R)-tert-butyl 2-(methoxycarbonylamino)-2-phenylacetate
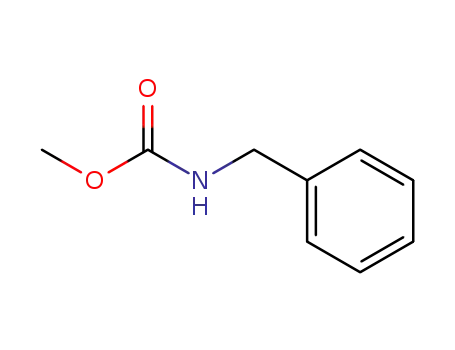
methyl N-benzylcarbamate
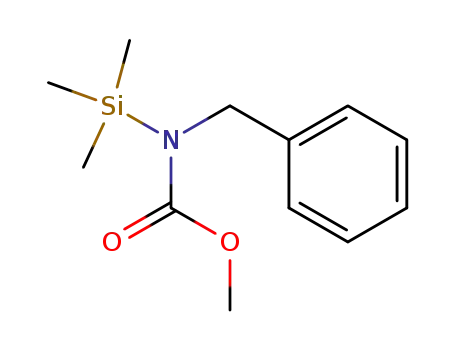
N-Benzyl-N-trimethylsilyl-carbamidsaeure-methylester
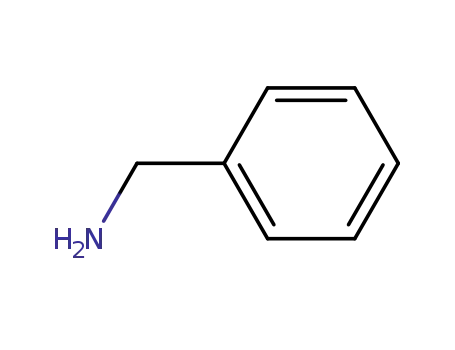
benzylamine
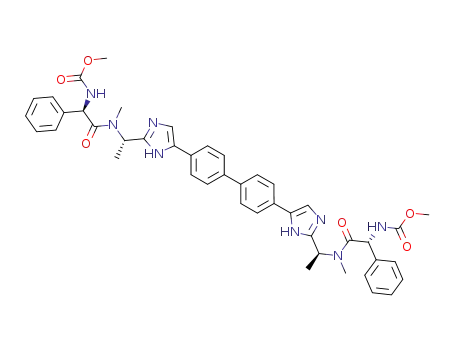
dimethyl (4,4'-biphenyldiylbis(1H-imidazole-5,2-diyl(1S)-1,1-ethanediyl(methylimino)((1R)-2-oxo-1-phenyl-2,1-ethanediyl)))biscarbamate
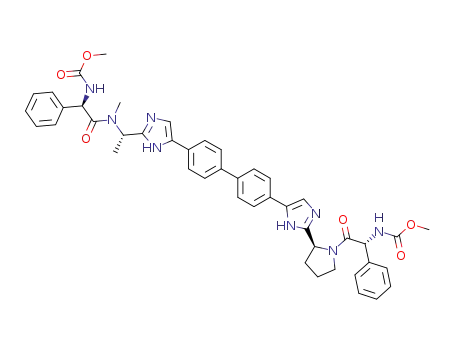
methyl ((1R)-2-((2S)-2-(5-(4'-(2-((1S)-1-(((2R)-2-((methoxy-carbonyl)amino)-2-phenyl-acetyl)(methyl)amino)ethyl)-1H-imidazol-5-yl)-4-biphenylyl)-1H-imidazol-2-yl)-1-pyrrolidinyl)-2-oxo-1-phenyl-ethyl)carbamate
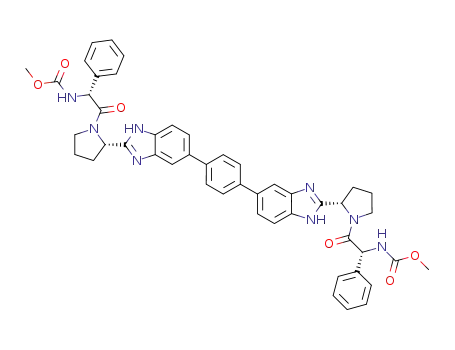
C48H46N8O6
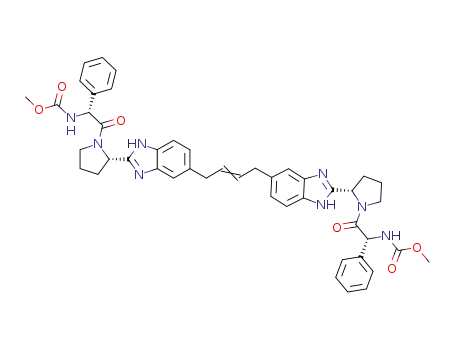
C46H48N8O6
CAS:56-12-2
CAS:7531-52-4
CAS:666832-71-9
CAS:17342-08-4