Your Location:Home >Products >Biochemical Engineering >4931-66-2
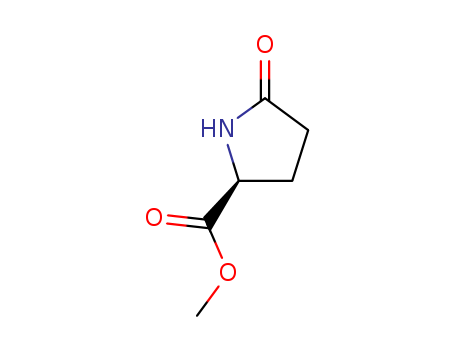

Product Details
|
Chemical Properties |
Brown Oil |
|
Uses |
Methyl (S)-Pyroglutamate (cas# 4931-66-2) is a compound useful in organic synthesis. |
InChI:InChI=1/C17H14F3N3S2/c1-21-16(23-15-4-2-3-13(9-15)17(18,19)20)25-10-12-5-7-14(8-6-12)22-11-24/h2-9H,10H2,1H3,(H,21,23)
An efficient methodology for the enantio...
Licochalcone A, as main constituent of C...
An organocatalytic method for the chemo-...
-
Some ways to use the N-acyl iminium salt...
Anthelvencins A and B are pyrrolamide me...
Twenty-eight 5-pyrrolidine-2-ones decora...
The enantioselective intermolecular cros...
Showdomycin is a C-nucleoside bearing an...
The straightforward synthesis of enantio...
CDK7 has emerged as an exciting target i...
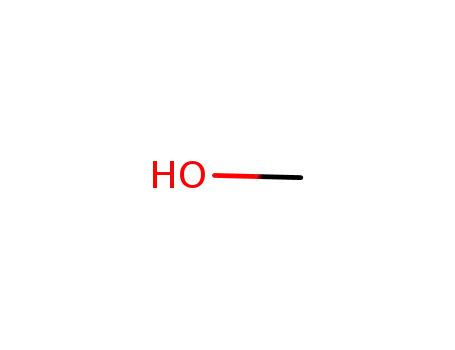
methanol

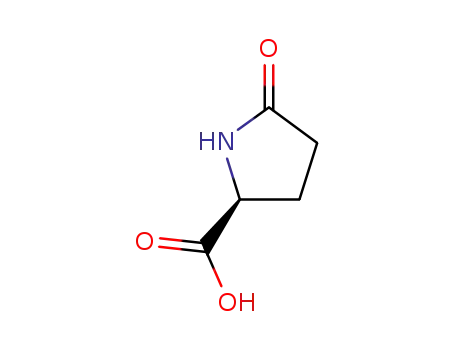
L-Pyroglutamic acid

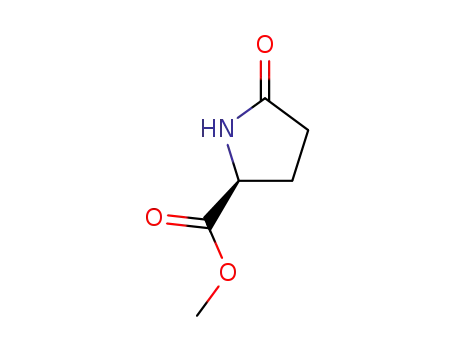
methyl (S)-pyroglutamate
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
at 0 - 25 ℃;
|
100% |
|
With
methanesulfonic acid;
In
chloroform; water;
Reflux;
|
100% |
|
With
Dowex-50W (X8-200) resin;
Inert atmosphere;
Reflux;
|
100% |
|
With
thionyl chloride;
for 0.5h;
Heating;
|
99.3% |
|
With
Amberlyst 15 resin;
for 24h;
Heating;
|
99% |
|
With
Dowex ion-exchange resin;
|
98% |
|
With
Dowex 50 W X 8;
for 4h;
Heating;
|
97% |
|
With
Amberlyst 15 wet resin;
for 24h;
Reflux;
|
97% |
|
With
Dowex;
for 16h;
Heating;
|
96% |
|
With
Dowex 50W X 8;
Heating;
|
95% |
|
With
thionyl chloride;
at 20 ℃;
for 48h;
|
95% |
|
With
thionyl chloride;
Ambient temperature;
|
94% |
|
With
thionyl chloride;
at -5 - 20 ℃;
for 10h;
|
94% |
|
With
thionyl chloride;
at 20 ℃;
for 12h;
|
94% |
|
With
thionyl chloride;
for 3h;
Ambient temperature;
|
93% |
|
With
Amberlyst 15;
Reflux;
|
93% |
|
With
thionyl chloride;
|
92% |
|
With
thionyl chloride;
at 0 - 20 ℃;
Inert atmosphere;
|
92% |
|
With
thionyl chloride;
at 20 ℃;
for 24h;
|
92% |
|
With
thionyl chloride;
In
N,N-dimethyl-formamide;
for 26h;
Ambient temperature;
|
91% |
|
With
thionyl chloride; N,N-dimethyl-formamide;
for 24h;
Ambient temperature;
|
91% |
|
With
thionyl chloride;
|
91% |
|
With
toluene-4-sulfonic acid;
for 12h;
Heating;
|
90% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 4h;
Inert atmosphere;
Schlenk technique;
|
90% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
|
90% |
|
With
thionyl chloride;
for 1.5h;
Ambient temperature;
|
89.1% |
|
With
thionyl chloride;
at 20 ℃;
for 24h;
|
85% |
|
With
thionyl chloride;
In
toluene;
at -15 - 20 ℃;
Inert atmosphere;
|
83% |
|
With
thionyl chloride;
for 72h;
|
81% |
|
With
thionyl chloride;
at -15 - 20 ℃;
Inert atmosphere;
|
81% |
|
With
thionyl chloride;
at 20 ℃;
for 16h;
Inert atmosphere;
|
81% |
|
With
thionyl chloride;
at -18 - 20 ℃;
|
81% |
|
With
thionyl chloride;
for 2h;
Ambient temperature;
|
80% |
|
With
thionyl chloride;
for 24h;
Ambient temperature;
|
80% |
|
With
thionyl chloride;
at 20 ℃;
for 3h;
pH=0;
|
80% |
|
With
thionyl chloride;
at 20 ℃;
|
80% |
|
With
thionyl chloride;
at 20 ℃;
for 14h;
|
79% |
|
With
thionyl chloride;
for 1.5h;
Heating;
|
76% |
|
With
thionyl chloride;
at 20 ℃;
Cooling with ice;
|
72% |
|
With
thionyl chloride;
at 20 ℃;
for 2h;
|
70% |
|
With
thionyl chloride;
at 0 - 20 ℃;
|
70% |
|
With
thionyl chloride;
at 0 - 20 ℃;
|
70% |
|
With
thionyl chloride;
at 0 ℃;
for 0.5h;
|
67% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
|
67.6% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
Inert atmosphere;
Sealed tube;
|
67.6% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
|
67.6% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
|
67.6% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
|
67.6% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
|
67.6% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
|
67.6% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 3h;
|
67.6% |
|
With
thionyl chloride;
at 0 - 40 ℃;
|
58% |
|
sulfuric acid;
In
benzene;
Heating;
|
|
|
sulfuric acid;
In
benzene;
for 20h;
Heating;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
for 2h;
Ambient temperature;
|
|
|
With
Dowex 50WX2-200;
for 3h;
Heating;
|
|
|
With
thionyl chloride;
at 20 ℃;
for 48h;
|
27.70 g |
|
With
thionyl chloride;
at -20 - 20 ℃;
|
|
|
With
thionyl chloride;
at -15 - 20 ℃;
|
|
|
With
thionyl chloride;
at 0 - 20 ℃;
|
|
|
With
thionyl chloride;
at -15 ℃;
for 2h;
|
|
|
With
thionyl chloride;
at -15 - 20 ℃;
for 2.5h;
|
|
|
With
hydrogenchloride;
at 20 ℃;
for 21h;
|
|
|
With
thionyl chloride;
at 10 - 30 ℃;
for 2.08333h;
|
|
|
With
thionyl chloride;
at 10 - 30 ℃;
for 2.08333h;
|
|
|
With
thionyl chloride;
at 0 - 23 ℃;
for 12h;
|
|
|
With
Dowex-H+;
Heating / reflux;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
at -15 - 20 ℃;
|
|
|
With
thionyl chloride;
at 20 ℃;
|
|
|
With
thionyl chloride;
In
methanol;
at 0 - 20 ℃;
for 2h;
|
|
|
With
hydrogenchloride;
In
water;
at 20 ℃;
for 24h;
|
|
|
With
toluene-4-sulfonic acid;
for 24h;
Reflux;
|
|
|
With
dmap; dicyclohexyl-carbodiimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
Inert atmosphere;
|
|
|
With
thionyl chloride;
at -5 - 30 ℃;
|
|
|
With
thionyl chloride;
at 20 ℃;
Cooling with ice;
Inert atmosphere;
|
|
|
With
thionyl chloride;
at 20 ℃;
for 1h;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
at 0 - 18 ℃;
for 1h;
|
|
|
With
toluene-4-sulfonic acid;
for 30h;
Reflux;
|
3.161 g |
|
With
thionyl chloride;
at 20 ℃;
for 1h;
Inert atmosphere;
|

L-Pyroglutamic acid


methyl (S)-pyroglutamate
| Conditions | Yield |
|---|---|
|
With
sulfuryl dichloride; sodium carbonate;
In
methanol; ethyl acetate;
|
92.8% |
|
With
sulfuryl dichloride; sodium carbonate;
In
methanol; ethyl acetate;
|
92.8% |
|
With
sulfuryl dichloride; sodium carbonate;
In
methanol; water; ethyl acetate; N,N-dimethyl-formamide;
|
91% |
|
With
sulfuryl dichloride; sodium carbonate;
In
methanol; water; ethyl acetate; N,N-dimethyl-formamide;
|
91% |
|
Multi-step reaction with 2 steps
1: SOCl2
With
thionyl chloride;
|
|
|
Multi-step reaction with 3 steps
1: 100 percent / saccharin / 2.5 h / Heating
2: 100 percent Chromat. / oxalyl chloride / CH2Cl2 / Heating
3: 86 percent / CH2Cl2 / 0.08 h / Heating
With
oxalyl dichloride; saccharin;
In
dichloromethane;
|
|
|
With
thionyl chloride; potassium carbonate;
In
methanol; water; ethyl acetate;
|
|
|
With
thionyl chloride;
In
methanol;
at 0 - 20 ℃;
for 3.5h;
|

methanol
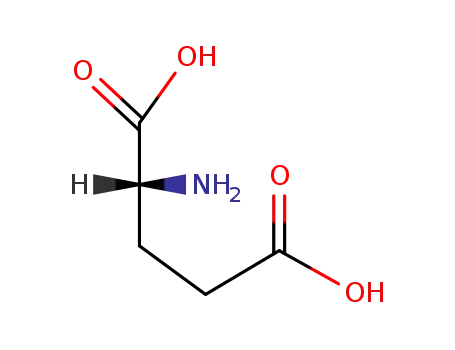
D-Glutamic acid
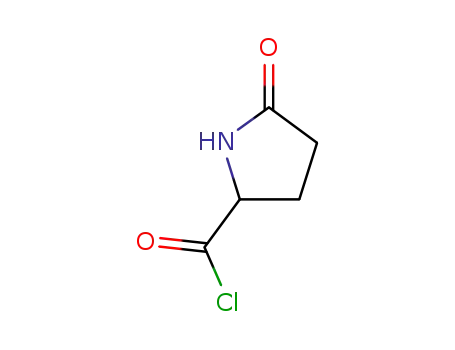
pyroglutamoyl chloride
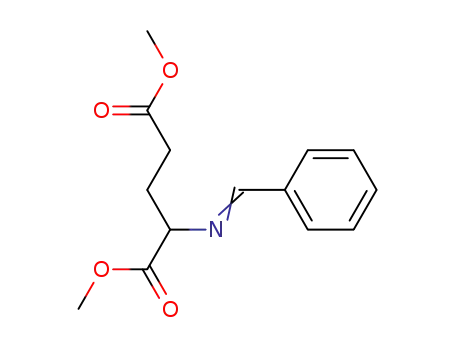
dimethyl N-benzylideneglutamate
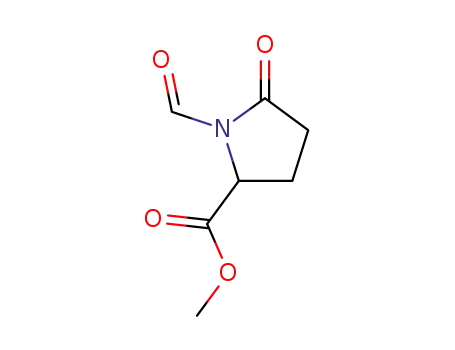
methyl N-formylpyroglutamate
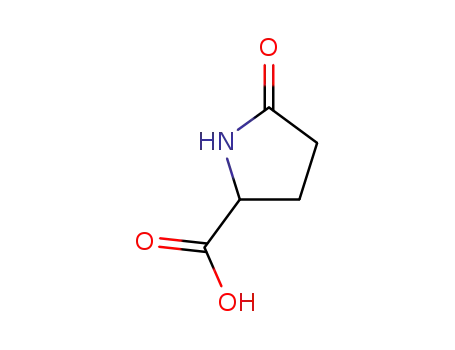
Pyroglutamic acid
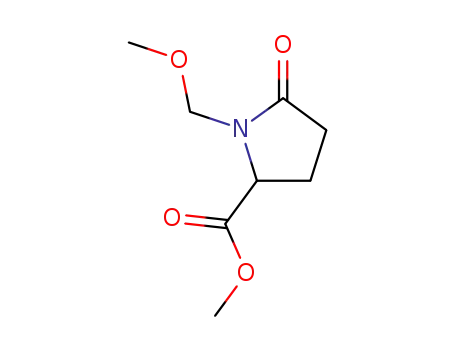
N-methoxy methyl pyroglutamate de methyle
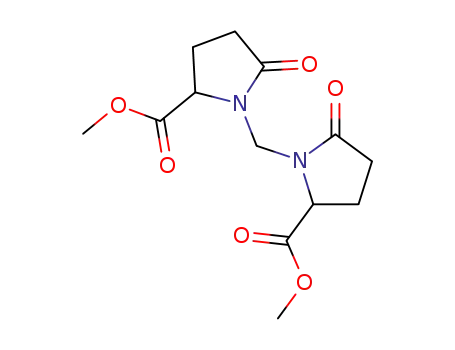
methyl methylene bis-pyroglutamate
CAS:56-12-2
CAS:7531-52-4
CAS:45120-30-7
CAS:666832-71-9