Your Location:Home >Products >Biochemical Engineering >1676-73-9
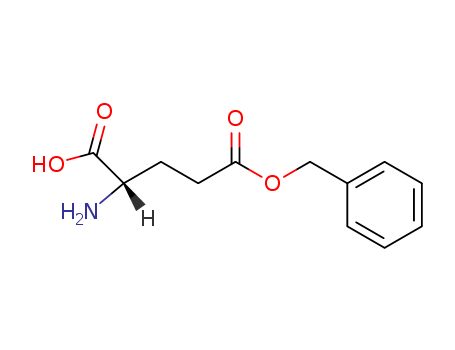

Product Details
|
Chemical Properties |
white powder |
|
Uses |
L-Glutamic acid γ-benzyl ester is commonly used in the synthesis of polymers for biological applications. Some of the examples are:Synthesis of bioreducible block copolymers based on poly(ethylene glycol) and poly(γ-benzyl L-glutamate) for Intracellular drug delivery.Synthesis of biodegradable poly(L-glutamic acid)-b-polylactide for magnetic resonance imaging (MRI)-visible drug delivery system.Synthesis of pH and temperature-responsive diblock copolymers based on poly(L-glutamic acid). |
|
Purification Methods |
Recrystallise the ester from H2O and store it at 0o. [Estrin Biochemical Preparations 13 25 1971, Beilstein 6 IV 2538.] |
InChI:InChI=1/C12H15NO4/c13-10(12(16)17)7-9(11(14)15)6-8-4-2-1-3-5-8/h1-5,9-10H,6-7,13H2,(H,14,15)(H,16,17)/p-1/t9-,10+/m1/s1
Novel smart microgel particles made of p...
The enzyme-catalyzed hydrolysis of diben...
The synthesis of boroxazolidones 1 from ...
Poly(ethylene glycol)-poly(l-glutamine) ...
We report the design and synthesis of ne...
Residual dipolar couplings (RDCs) have r...
-
RNA interfere (RNAi)-based technology ho...
Schiff base vanadium(IV) complexes of ph...
Muramyl dipeptide (MDP) is the smallest ...
The invention provides a preparation met...
Rhamnolipids are biodegradable low toxic...
Disclosed are compounds having potency i...
L-glutamic acid

benzyl alcohol

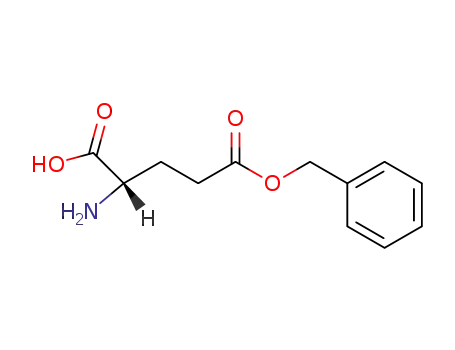
L-glutamic acid γ-benzyl ester
| Conditions | Yield |
|---|---|
|
With
tetrafluoroboric acid diethyl ether; sodium sulfate;
for 15h;
Ambient temperature;
|
94% |
|
With
tetrafluoroboric acid diethyl ether; sodium sulfate;
at 20 ℃;
for 12h;
Inert atmosphere;
|
80% |
|
L-glutamic acid; benzyl alcohol;
With
methanesulfonic acid;
In
toluene;
at 30 - 45 ℃;
for 6h;
With
ammonia;
In
ethanol; water;
at 60 ℃;
for 2h;
pH=6.5 - 7;
|
77% |
|
With
methanesulfonic acid;
In
toluene;
at 0 - 40 ℃;
|
71% |
|
With
methanesulfonic acid;
In
toluene;
at 30 - 45 ℃;
for 5h;
Inert atmosphere;
|
67% |
|
benzyl alcohol;
With
sulfuric acid;
In
diethyl ether;
L-glutamic acid;
at 20 ℃;
With
pyridine;
In
ethanol;
for 1h;
chemoselective reaction;
Cooling with ice;
|
60% |
|
With
sulfuric acid;
In
diethyl ether;
for 20h;
Ambient temperature;
|
59% |
|
With
sulfuric acid;
at 70 ℃;
for 4h;
|
59% |
|
With
toluene-4-sulfonic acid;
In
toluene;
at 30 - 50 ℃;
for 5h;
|
55% |
|
With
sulfuric acid;
In
diethyl ether;
|
50% |
|
L-glutamic acid; benzyl alcohol;
In
toluene;
at 45 ℃;
for 2h;
With
methanesulfonic acid;
In
toluene;
at 20 - 45 ℃;
for 3h;
|
49.63% |
|
With
hydrogen bromide;
In
tetrahydrofuran;
at 70 ℃;
|
44.1% |
|
With
sulfuric acid;
In
water;
at 70 ℃;
for 4.75h;
|
33% |
|
With
hydrogenchloride;
1.) 100 deg C, 3 h, 2.) r.t., overnight;
|
30% |
|
With
toluene-4-sulfonic acid;
at 100 - 105 ℃;
for 0.416667h;
|
30% |
|
With
pyridine; hydrogen bromide;
In
ethanol;
at 70 ℃;
for 1.5h;
|
25.2% |
|
With
methanesulfonic acid;
In
toluene;
at 30 - 45 ℃;
for 6h;
|
21% |
|
With
sulfuric acid;
|
|
|
With
hydrogen iodide;
|
|
|
With
hydrogenchloride;
|
|
|
|
|
|
With
hydrogen iodide;
|
|
|
With
toluene-4-sulfonic acid;
|
|
|
With
sulfuric acid;
In
diethyl ether;
|
|
|
L-glutamic acid; benzyl alcohol;
With
sulfuric acid;
With
pyridine;
In
ethanol;
Further stages.;
|
|
|
With
sulfuric acid;
|
|
|
benzyl alcohol;
With
sulfuric acid;
In
diethyl ether;
at 20 ℃;
for 0.25h;
L-glutamic acid;
With
triethylamine;
for 20h;
pH=7-8;
|
75 g |
|
With
sulfuric acid;
In
water;
at 70 ℃;
for 0.5h;
|
|
|
L-glutamic acid;
With
sulfuric acid;
In
diethyl ether;
at 10 - 20 ℃;
for 6h;
benzyl alcohol;
In
diethyl ether;
|
104 g |
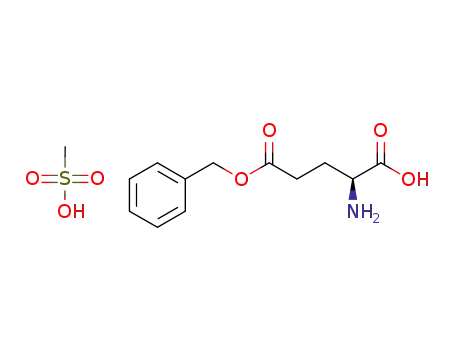
γ-benzyl L-glutamate methanesulphonate


L-glutamic acid γ-benzyl ester
| Conditions | Yield |
|---|---|
|
With
ammonia;
In
water;
at 13 - 15 ℃;
for 2h;
pH=5.9 - 6.3;
|
81% |

L-glutamic acid
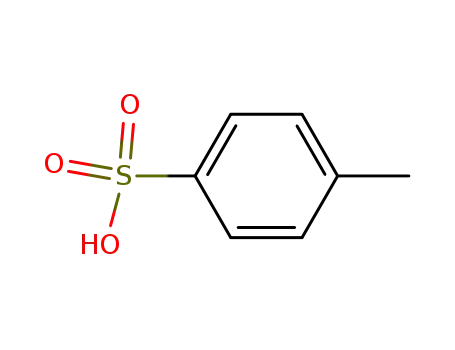
toluene-4-sulfonic acid

benzyl alcohol
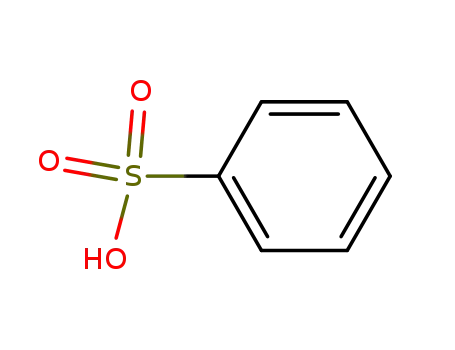
benzenesulfonic acid
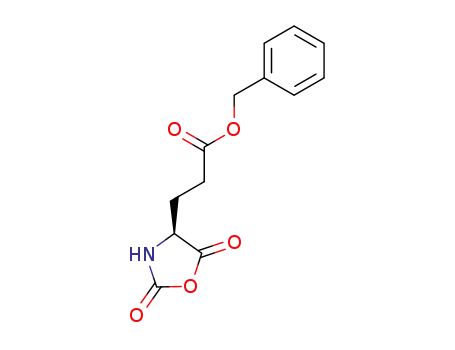
5-benzyl L-glutamate N-carboxyanhydride
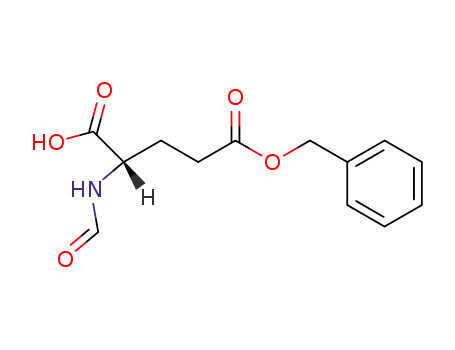
N-formyl-L-glutamic acid-5-benzyl ester
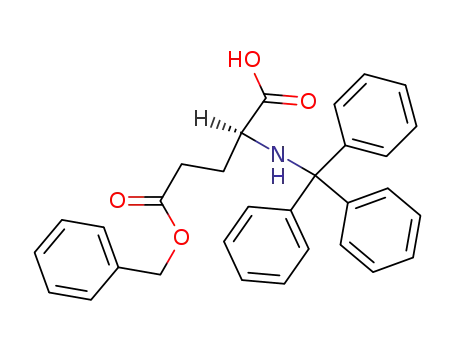
N-trityl-L-glutamic acid-5-benzyl ester
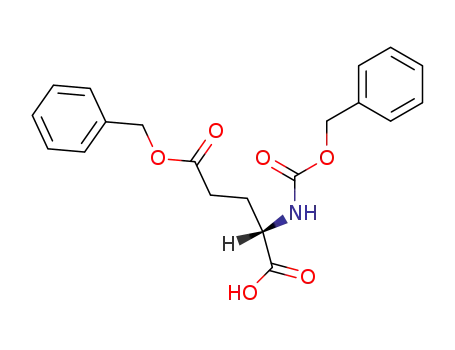
N-benzyloxycarbonyl-5-O-benzyl-L-glutamic acid
CAS:56-12-2
CAS:7531-52-4
CAS:13726-85-7
CAS:45120-30-7