Your Location:Home >Products >Biochemical Engineering >13726-85-7
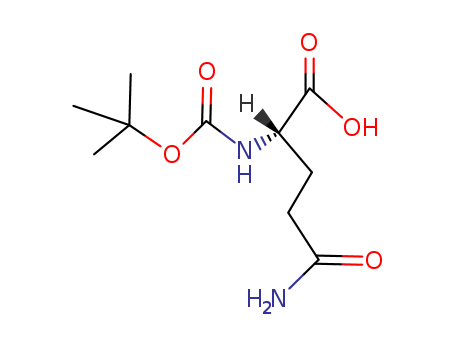

Product Details
|
Chemical Properties |
White fine crystalline powder |
|
Uses |
Protected Glutamine. |
InChI:InChI=1/C10H18N2O5/c1-10(2,3)17-9(16)12-6(8(14)15)4-5-7(11)13/h6H,4-5H2,1-3H3,(H2,11,13)(H,12,16)(H,14,15)/p-1/t6-/m0/s1
Bimodal PNAs are new PNA constructs desi...
Cα-bimodal peptide nucleic acids (bm-Cα-...
The present invention relates to a proce...
Peptide nucleic acids (PNAs) are linear ...
L-glutamine

di-tert-butyl dicarbonate

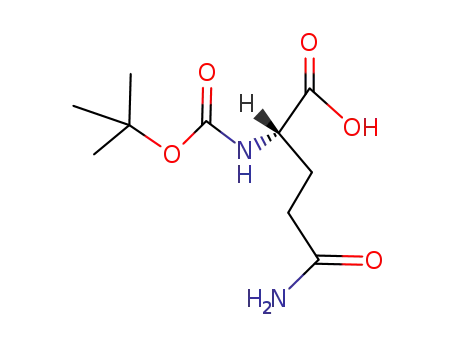
Boc-Gln-OH
| Conditions | Yield |
|---|---|
|
|
95% |
|
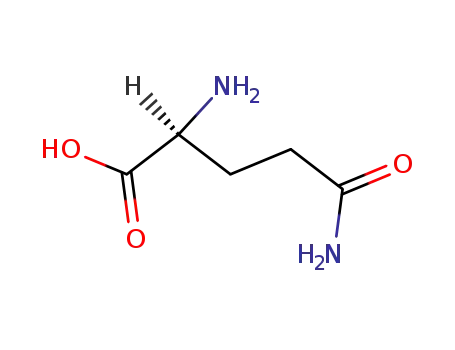
L-glutamine;
With
sodium hydroxide;
In
1,4-dioxane; water;
Large scale;
di-tert-butyl dicarbonate;
In
1,4-dioxane;
at 0 - 20 ℃;
for 4h;
Reagent/catalyst;
Solvent;
Temperature;
Large scale;
|
90.7% |
|
With
sodium hydroxide;
In
1,4-dioxane;
for 1h;
|
90% |
|
With
sodium hydroxide;
In
tetrahydrofuran; water;
at 10 - 25 ℃;
for 20h;
|
89% |
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 5 - 20 ℃;
for 4.5h;
|
87% |
|
With
sodium hydroxide;
In
1,4-dioxane;
for 1h;
|
86% |
|
With
sodium hydroxide;
In
1,4-dioxane;
at 0 - 20 ℃;
for 4.5h;
|
85% |
|
With
sodium hydroxide;
In
water; acetonitrile;
at 20 - 40 ℃;
for 18h;
pH=8;
|
85% |
|
With
sodium hydroxide;
In
1,4-dioxane;
for 1h;
Ambient temperature;
|
70% |
|
In
water; tert-butyl alcohol;
pH 9.5-10.0;
|
|
|
With
sodium hydroxide; water;
In
tetrahydrofuran;
at 20 ℃;
for 5h;
|
|
|
With
triethylamine;
In
1,4-dioxane; water;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
Inert atmosphere;
|
|
|
With
sodium hydroxide;
at 0 - 20 ℃;
|
|
|
With
sodium carbonate;
In
acetone;
at 20 ℃;
pH=8-9;
Large scale;
|
1.3 kg |
|
With
sodium hydroxide;
In
water;
at 20 ℃;
|
|
|
With
sodium hydroxide;
In
1,4-dioxane;
for 1h;
|
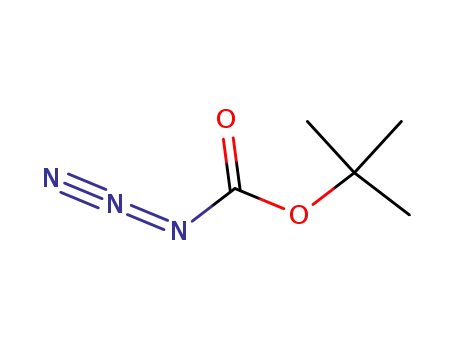
N-(tert-butyloxycarbonyl) azide


L-glutamine


Boc-Gln-OH
| Conditions | Yield |
|---|---|
|
|
|
|
With
triethylamine;
In
1,4-dioxane; water;
|
|
|
With
triethylamine;
|
|
|
With
triethylamine;
In
water; N,N-dimethyl-formamide;
|

N-(tert-butyloxycarbonyl) azide

L-glutamine
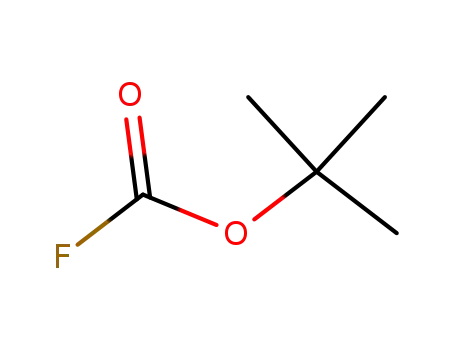
tert-butyl fluoroformate
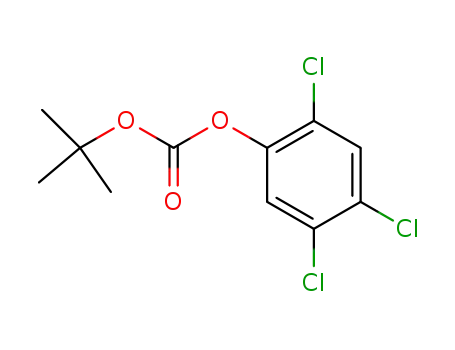
1,1-dimethylethyl 2,4,5-trichlorophenyl carbonate
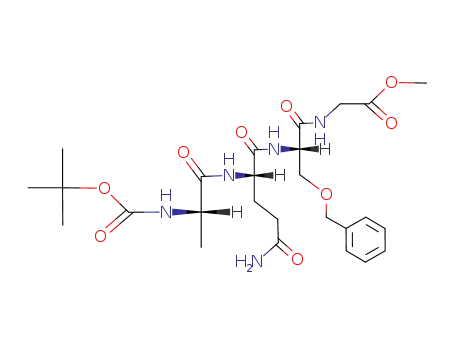
Boc-Ala-Gln-Ser(Bzl)-Gly-OCH3
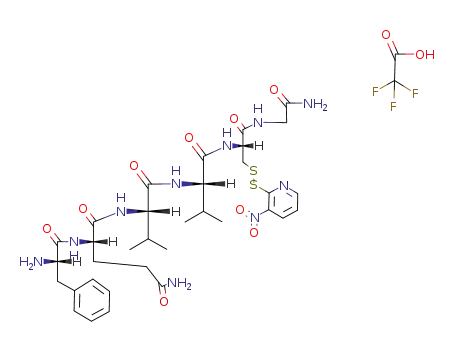
TFA*H-Phe-Gln-Val-Val-Cys-S-(3-nitro-2-pyridinesulfenyl)-Gly-NH2
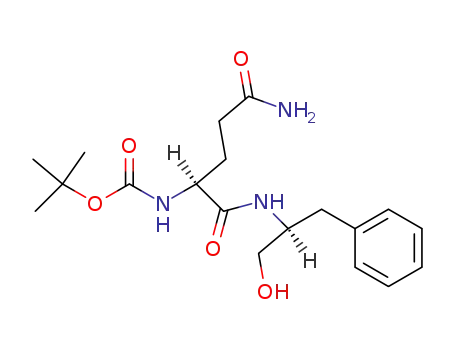
Boc-Gln-Phol
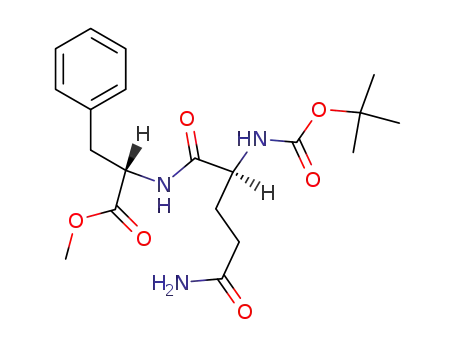
Boc-Gln-Phe-OMe
CAS:56-12-2
CAS:7531-52-4
CAS:683239-16-9
CAS:1676-73-9