Your Location:Home >Products >683239-16-9
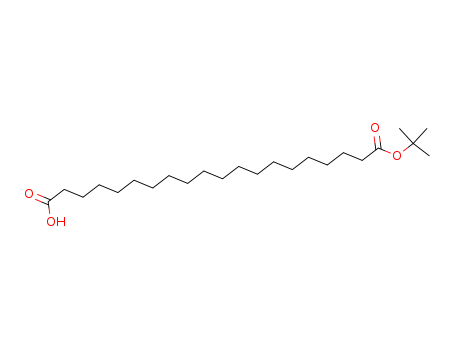

Product Details
|
Description |
20-(tert-Butoxy)-20-oxoicosanoic acid is a non-cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs) and in the synthesis of PROTACs. |
|
Uses |
20-(tert-Butoxy)-20-oxoicosanoic acid is a useful research chemical compound. |
The invention provides a method for obta...
Recently, the first basal oral insulin (...
The invention discloses a method for pre...
The present invention relates to novel c...
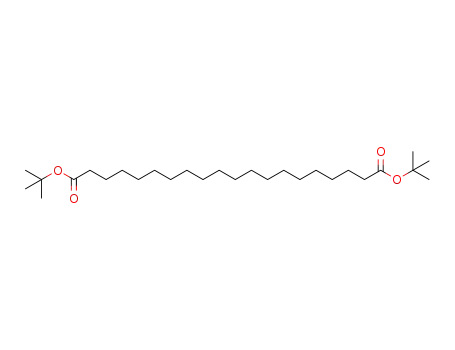
tert-butyl icosanedioic acid

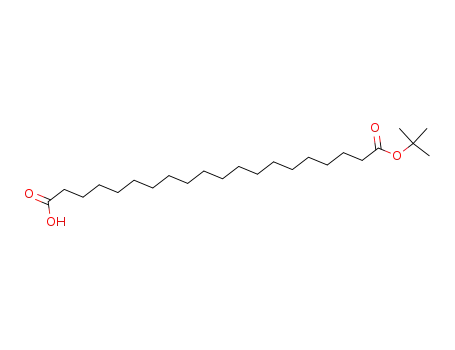
eicosanedioic acid mono(1,1-dimethylethyl)ester

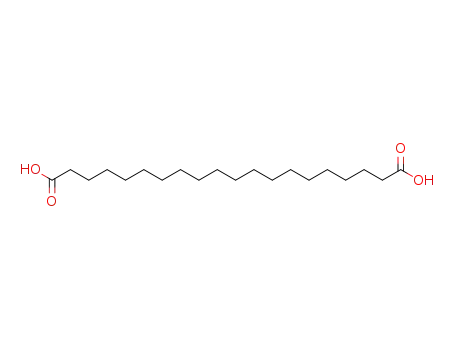
octadecane-1,18-dicarboxylic acid

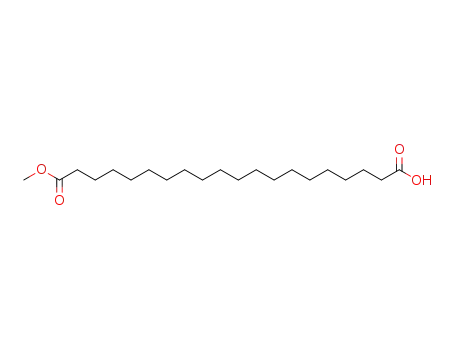
20-methoxy-20-oxoicosanoic acid
| Conditions | Yield |
|---|---|
|
With
methanol; barium hydroxide octahydrate;
at 30 - 35 ℃;
for 14.5h;
|
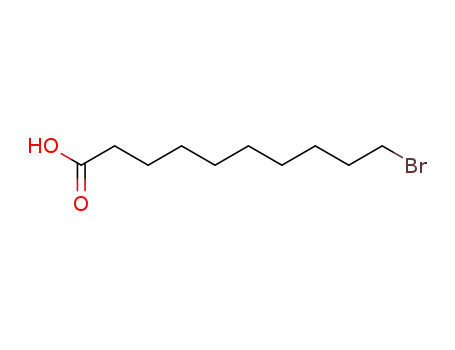
10-bromodecanoic acid


eicosanedioic acid mono(1,1-dimethylethyl)ester


octadecane-1,18-dicarboxylic acid


20-methoxy-20-oxoicosanoic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: trifluoroacetic anhydride / dichloromethane / 0.5 h / 0 °C / Large scale
1.2: 2 h / 0 - 10 °C / Large scale
2.1: zinc; nickel(II) chloride hexahydrate; 2,2':6,2''-terpyridine / N,N-dimethyl-formamide / 10 h / 35 - 45 °C / Inert atmosphere; Large scale
3.1: barium hydroxide octahydrate; methanol / 14.5 h / 30 - 35 °C
With
methanol; 2,2':6,2''-terpyridine; nickel(II) chloride hexahydrate; barium hydroxide octahydrate; trifluoroacetic anhydride; zinc;
In
dichloromethane; N,N-dimethyl-formamide;
|

octadecane-1,18-dicarboxylic acid
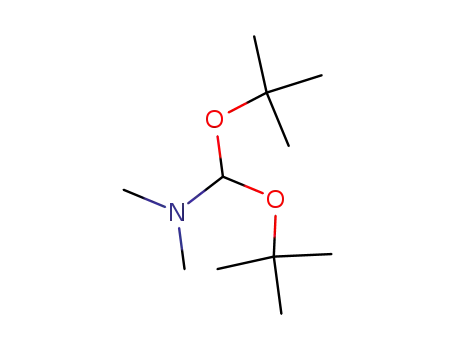
N,N-dimethylformamide di-tert-butyl acetal
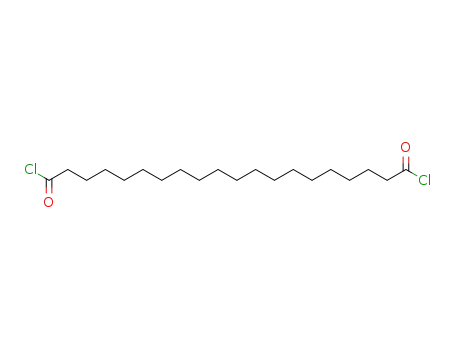
1,20-eicosanedioyl dichloride
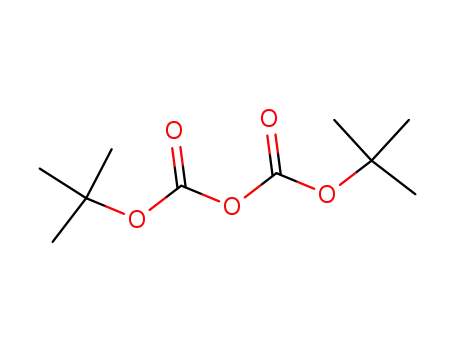
di-tert-butyl dicarbonate
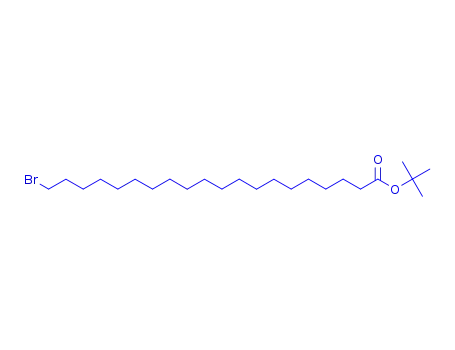
20-bromo-icosanoic acid tert-butyl ester
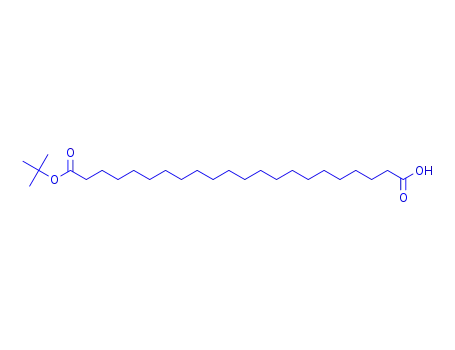
docosanedioic acid mono-tert-butyl ester
CAS:56-12-2
CAS:7531-52-4
CAS:166170-15-6
CAS:13726-85-7