Your Location:Home >Products >166170-15-6
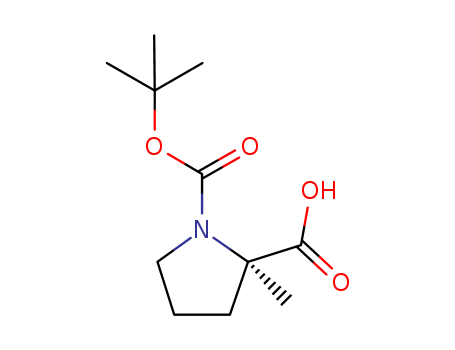

Product Details
The present disclosure relates to compou...
(R)-Boc-2-methylproline (3a) was synthes...
The invention discloses a synthetic meth...
The present invention provides a dihydro...
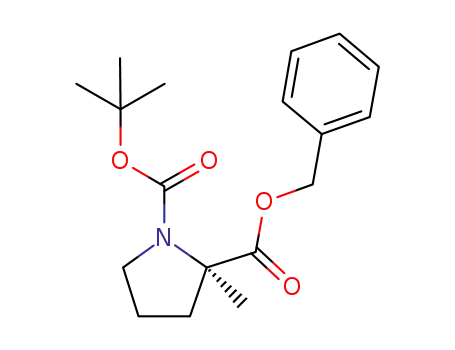
(R)-2-benzyl 1-tert-butyl 2-methylpyrrolidine-1,2-dicarboxylate

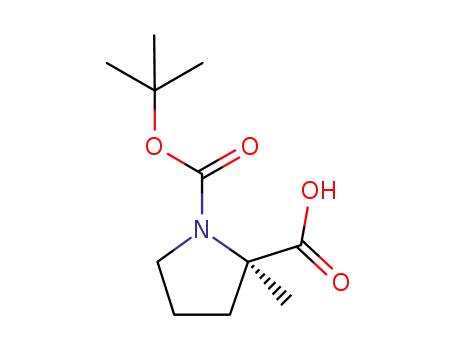
(R)-1-(tert-butoxycarbonyl)-2-methylpyrrolidine-2-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
5%-palladium/activated carbon; hydrogen;
In
ethanol;
at 20 ℃;
under 2828.7 Torr;
Inert atmosphere;
|
99.3% |
|
With
hydrogen;
5%-palladium/activated carbon;
In
ethanol; Isopropyl acetate;
at 40 ℃;
under 2068.65 Torr;
|
97% |
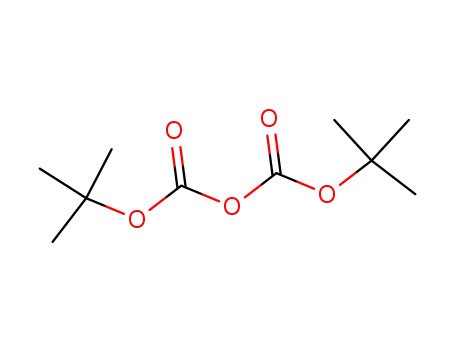
di-tert-butyl dicarbonate

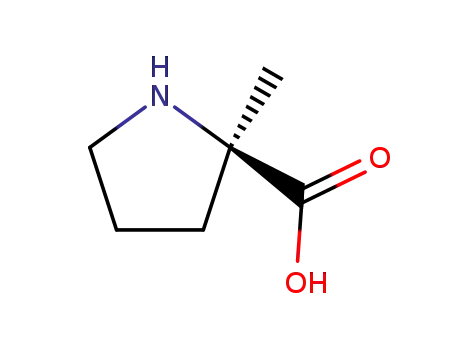
(R)-2-Methyl-1-azacyclopentan-2-carbonsaeure


(R)-1-(tert-butoxycarbonyl)-2-methylpyrrolidine-2-carboxylic acid
| Conditions | Yield |
|---|---|
|
With
tetramethyl ammoniumhydroxide;
In
acetonitrile;
at 20 - 40 ℃;
|
93% |
|
With
sodium carbonate;
In
1,4-dioxane; water;
at 0 - 20 ℃;
|
74% |
|
With
sodium hydroxide;
In
1,4-dioxane; water;
at 0 - 20 ℃;
for 2h;
|
|
|
With
triethylamine;
In
water; acetonitrile;
at 25 ℃;
for 10h;
|

(R)-2-benzyl 1-tert-butyl 2-methylpyrrolidine-1,2-dicarboxylate

di-tert-butyl dicarbonate

(R)-2-Methyl-1-azacyclopentan-2-carbonsaeure
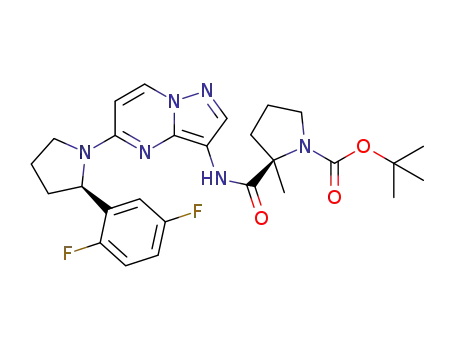
(R)-tert-butyl 2-(5-((R)-2-(2,5-difluorophenyl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidin-3-ylcarbamoyl)-2-methylpyrrolidine-1-carboxylate
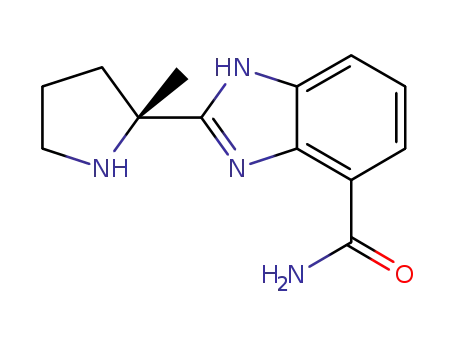
veliparib
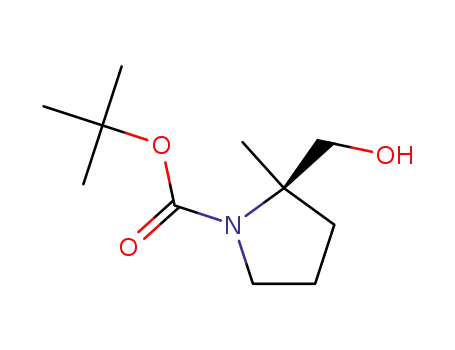
tert-butyl (R)-2-(hydroxymethyl)-2-methylpyrrolidine-1-carboxylate
CAS:56-12-2
CAS:7531-52-4
CAS:309956-78-3
CAS:683239-16-9