Your Location:Home >Products >Pharmaceutical intermediates >309956-78-3
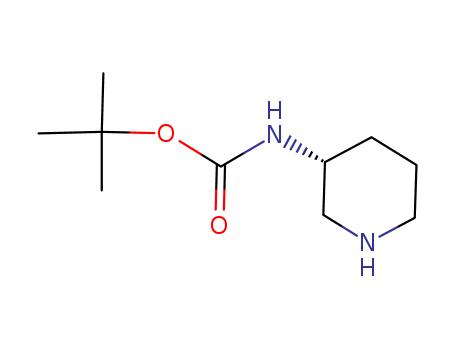

Product Details
|
Solubility in organics |
Soluble in methanol and ethanol. |
|
Uses |
(R)-3-(Boc-amino)piperidine is used as an organic chemical synthesis intermediate. |
|
storage |
Stable under recommended storage conditions. Incompatible with oxidizing agents. |
|
Chemical Properties |
White powder |
InChI:InChI=1/C10H20N2O2/c1-10(2,3)14-9(13)12-8-5-4-6-11-7-8/h8,11H,4-7H2,1-3H3,(H,12,13)/t8-/m1/s1
(R)-3-[(tert -Butoxycarbonyl)amino]piper...
The invention relates to a synthesis met...
The invention discloses a preparation me...
The invention discloses a preparation me...
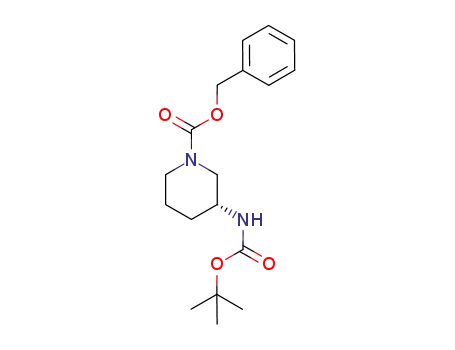
(R)-3-(tert-butoxycarbonylamino)piperidine-1-carboxylic acid benzyl ester

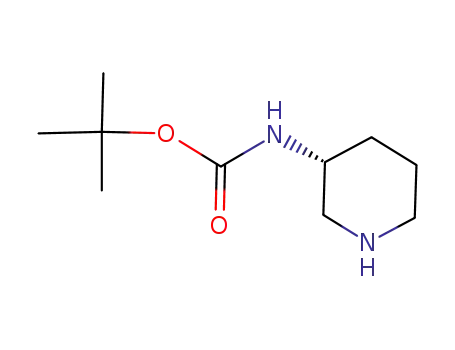
(R)-piperidin-3-ylcarbamic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol; water;
at 35 - 40 ℃;
for 2h;
under 2250.23 - 3000.3 Torr;
Time;
Autoclave;
Inert atmosphere;
|
95.4% |
|
With
hydrogen;
palladium 10% on activated carbon;
In
ethanol;
at 20 ℃;
for 168h;
under 760.051 Torr;
|
92% |
|
In
ethanol;
|
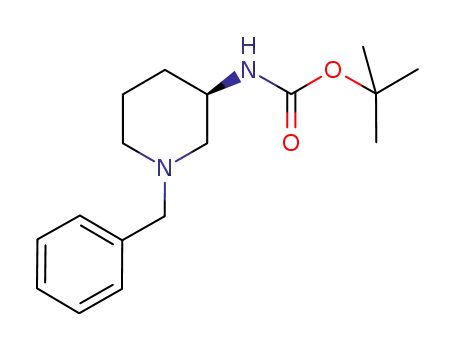
tert-butyl (R)-(+)-(N-benzylpiperidin-3-yl)carbamate


(R)-piperidin-3-ylcarbamic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With
5%-palladium/activated carbon; hydrogen;
In
ethanol;
at 20 - 25 ℃;
for 20h;
under 150015 Torr;
|
97% |
|
With
palladium 10% on activated carbon; hydrogen;
In
methanol;
at 45 ℃;
for 2h;
under 1140.08 Torr;
|
85% |
|
With
hydrogen;
10% palladium on activated carbon;
In
metahnol;
at 20 ℃;
for 24h;
|

(R)-3-(tert-butoxycarbonylamino)piperidine-1-carboxylic acid benzyl ester

tert-butyl (R)-(+)-(N-benzylpiperidin-3-yl)carbamate
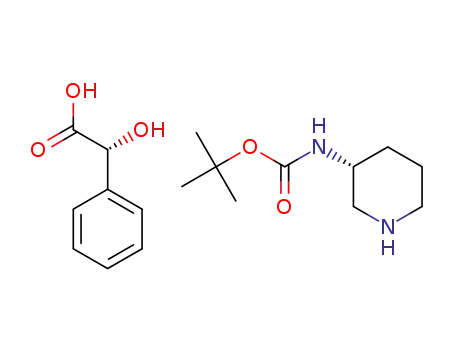
t-butyl (R)-piperidin-3-ylcarbamate R-mandelate
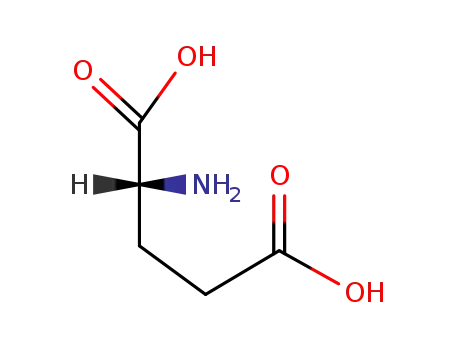
D-Glutamic acid
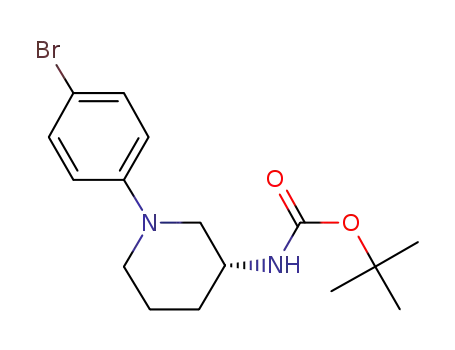
[(R)-1-(4-bromo-phenyl)-piperidin-3-yl]-carbamic acid-tert-butylester
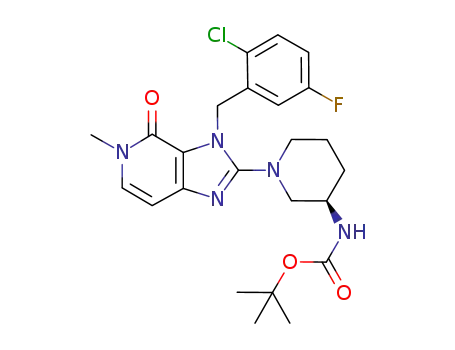
tert-butyl {(3R)-1-[3-(2-chloro-5-fluorobenzyl)-5-methyl-4-oxo-4,5-dihydro-3H-imidazo[4,5-c]pyridin-2-yl]piperidin-3-yl}carbamate
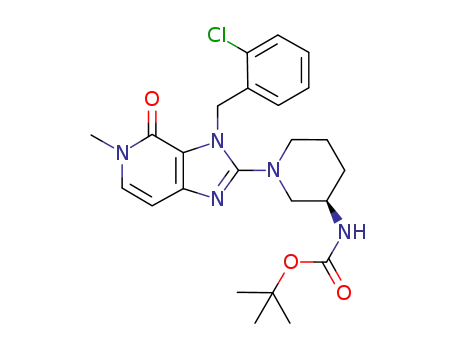
tert-butyl {(3R)-1-[3-(2-chlorobenzyl)-5-methyl-4-oxo-4,5-dihydro-3H-imidazo[4,5-c]pyridin-2-yl]piperidin-3-yl}carbamate
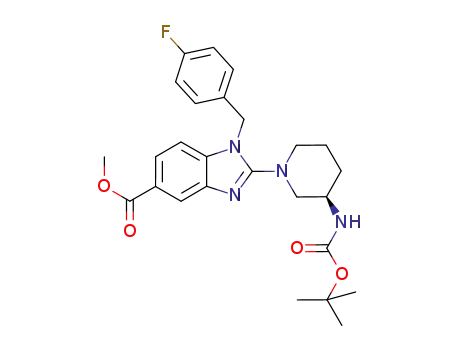
R-2-(3-tert-butoxycarbonylamino-piperidin-1-yl)-1-(4-fluoro-benzyl)-1H-benzimidazole-5-carbonic acid methyl ester
CAS:56-12-2
CAS:7531-52-4
CAS:147081-29-6
CAS:166170-15-6