Your Location:Home >Products >147081-29-6

Product Details
|
Chemical Properties |
colorless to yellow liquid |
|
Uses |
Laboratory chemicals, Manufacture of substances. |
InChI:InChI=1/C10H20N2O2/c1-8-7-12(6-5-11-8)9(13)14-10(2,3)4/h8,11H,5-7H2,1-4H3/t8-/m0/s1
The present application relates to compo...
The present application relates to compo...
Efforts toward the process development o...
Methods for preparing the Bruton's Tyros...
di-tert-butyl dicarbonate

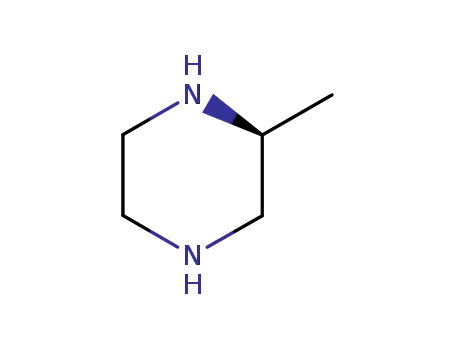
(S)-2-methylpiperazine

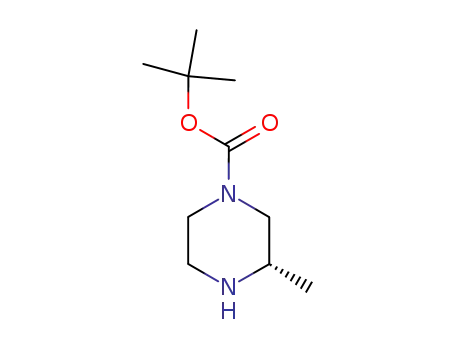
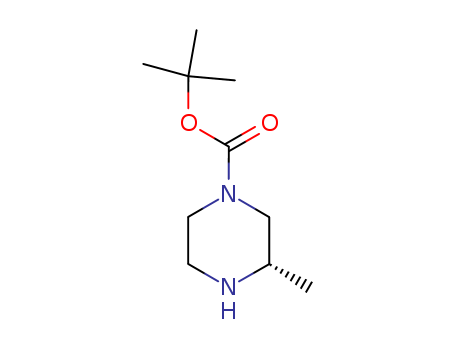
(S)-3-methyl-piperazine-1-carboxylic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With
N-ethyl-N,N-diisopropylamine;
In
ethanol;
at 20 ℃;
for 16h;
|
90% |
|
With
N-ethyl-N,N-diisopropylamine;
In
ethanol;
at 20 ℃;
for 16h;
|
90% |
|
With
hydrogenchloride;
In
methanol; water;
at 15 - 20 ℃;
for 16h;
Inert atmosphere;
Industrial scale;
|
86% |
|
In
dichloromethane;
at 20 ℃;
for 4h;
|
84% |
|
In
dichloromethane;
at 0 - 20 ℃;
for 4h;
|
84% |
|
With
triethylamine;
In
dichloromethane;
for 5h;
|
80% |
|
With
hydrogenchloride;
In
methanol; water;
at 15 - 30 ℃;
for 18h;
Temperature;
Solvent;
|
76.4% |
|
In
dichloromethane;
at 0 - 20 ℃;
|
49% |
|
With
triethylamine;
In
methanol;
for 17h;
|
|
|
With
triethylamine;
In
hexane; dichloromethane; ethyl acetate;
|
|
|
With
triethylamine;
In
methanol;
for 17h;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 ℃;
for 2h;
|
562 mg |
|
With
triethylamine;
In
hexane; dichloromethane;
|

(S)-2-methylpiperazine

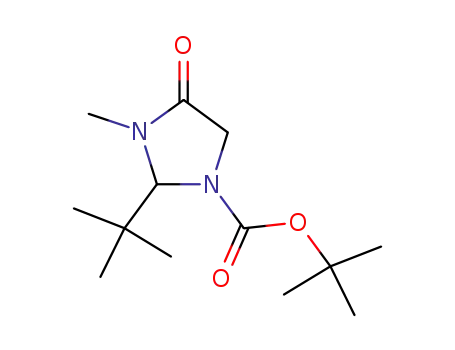
(S)-(-)1-(t-butoxycarbonyl)-2-t-butyl-3-methyl-4-imidazolidinone


(S)-3-methyl-piperazine-1-carboxylic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With
triethylamine;
In
chloroform;
at 20 ℃;
for 15h;
|
|
|
With
triethylamine;
In
chloroform;
|
|
|
With
triethylamine;
In
chloroform;
|
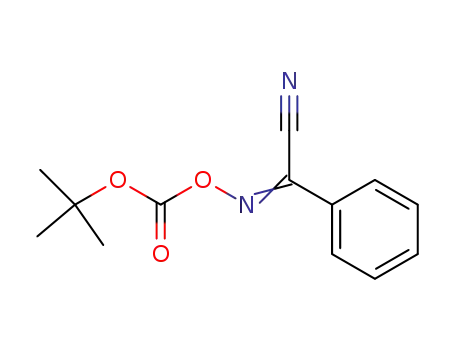
2-(tert-Butoxycarbonyloxyimino)-2-phenylacetonitrile

(S)-2-methylpiperazine
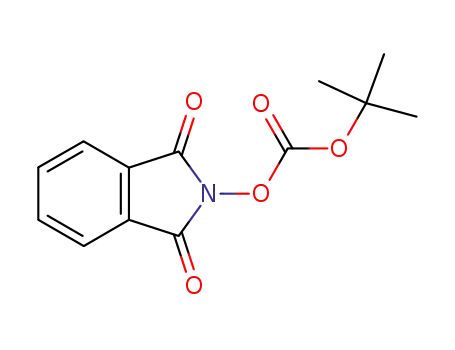
N-(t-butoxycarbonyloxy)-phthalimide

(S)-(-)1-(t-butoxycarbonyl)-2-t-butyl-3-methyl-4-imidazolidinone
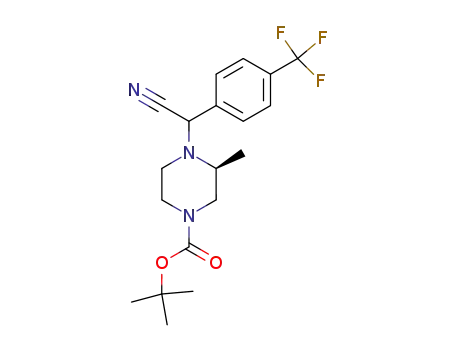
4-[cyano-(4-trifluoromethyl-phenyl)-methyl]-3-methyl-piperazine-1-carboxylic acid tert-butyl ester
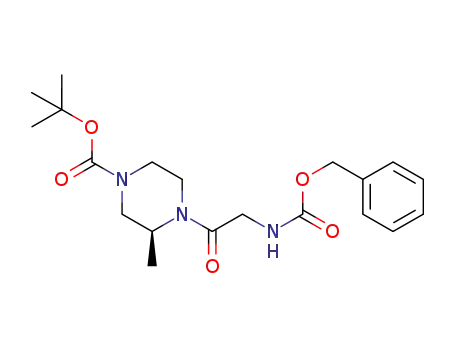
C20H29N3O5
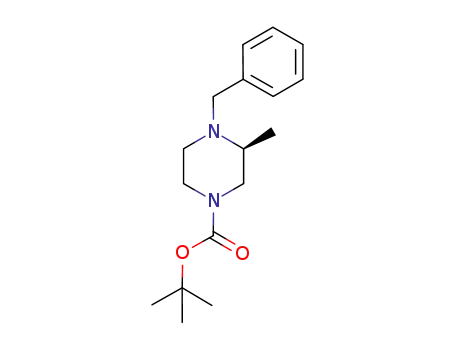
tert-butyl (3S)-4-benzyl-3-methylpiperazine-1-carboxylate
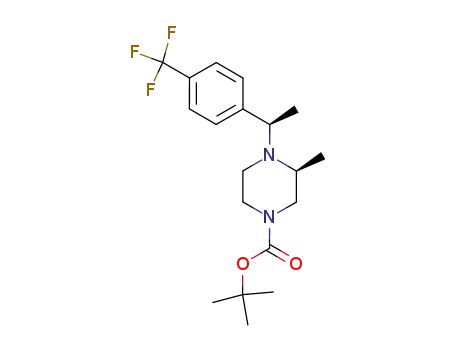
(S)-3-Methyl-4-[(R)-1-(4-trifluoromethyl-phenyl)-ethyl]-piperazine-1-carboxylic acid tert-butyl ester
CAS:56-12-2
CAS:7531-52-4
CAS:163765-44-4
CAS:309956-78-3