Your Location:Home >Products >163765-44-4
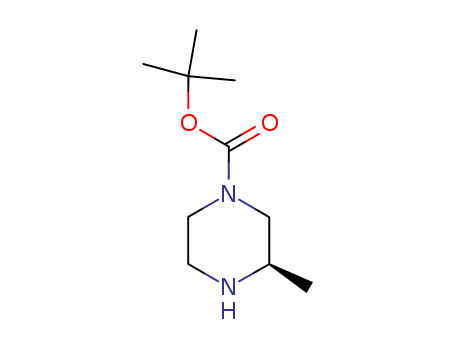

Product Details
|
Chemical Properties |
White solid |
|
Uses |
(R)-4-Boc-2-methylpiperazine is a reagent used in organic synthesis. Used in the synthesis of orally bioavailable inhibitors of 11-β-hydroxysteroid |
The invention provides a preparation met...
The invention provides a preparation met...
1,n-Metal shift is an elegant alternativ...
The invention discloses a novel method o...
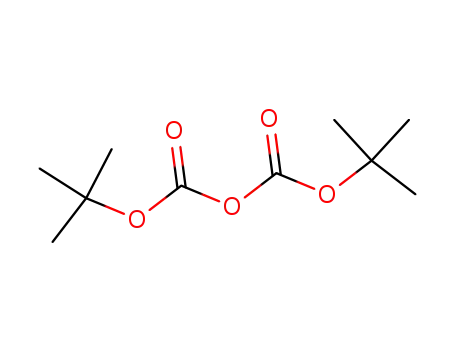
di-tert-butyl dicarbonate

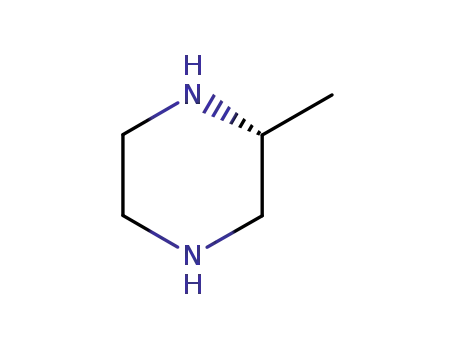
(2R)-methylpiperazine

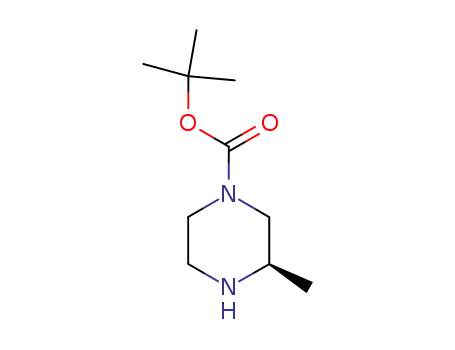
(R)-tert-butyl 3-methylpiperazine-1-carboxylate
| Conditions | Yield |
|---|---|
|
In
dichloromethane;
at 0 - 20 ℃;
for 4h;
|
84% |
|
In
dichloromethane;
at 0 - 20 ℃;
|
50% |
|
In
dichloromethane;
at 0 - 20 ℃;
|
50% |
|
In
tetrahydrofuran;
at 20 ℃;
for 12h;
|
48% |
|
With
triethylamine;
In
hexane; dichloromethane; ethyl acetate;
|
|
|
In
tetrahydrofuran;
at 20 ℃;
for 18h;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
for 1.75h;
|
|
|
With
triethylamine;
In
hexane; dichloromethane;
|
|
|
In
chloroform;
for 1h;
|
|
|
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
In
dichloromethane;
at 0 ℃;
for 1h;
|
25 g |
|
In
dichloromethane;
at 0 ℃;
for 1h;
|
26 g |
|
In
dichloromethane;
at 0 ℃;
for 1h;
|
25.4 g |

(2R)-methylpiperazine


(R)-tert-butyl 3-methylpiperazine-1-carboxylate
| Conditions | Yield |
|---|---|
|
|
96% |

(2R)-methylpiperazine

di-tert-butyl dicarbonate
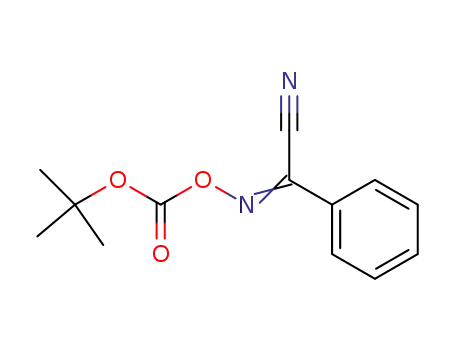
2-(tert-Butoxycarbonyloxyimino)-2-phenylacetonitrile
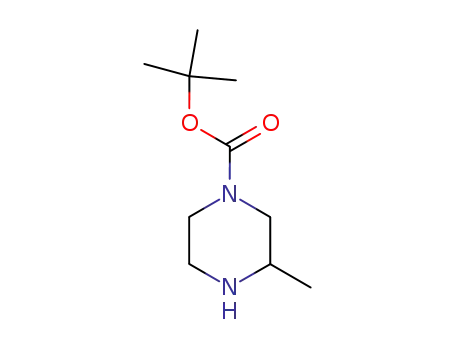
3-methyl-piperazine-1-carboxylic acid tert-butyl ester
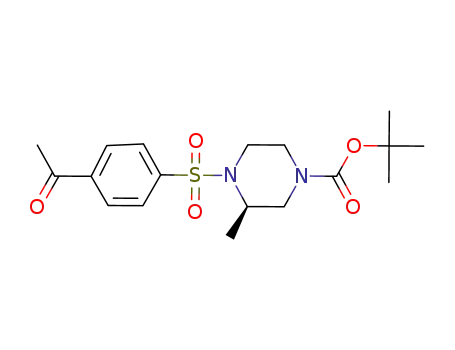
(R)-tert-butyl 4-(4-acetylphenylsulfonyl)-3-methylpiperazine-1-carboxylate
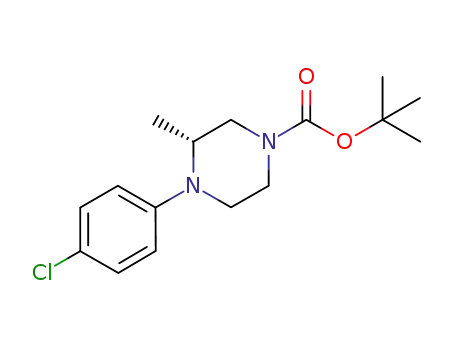
4-(4-chloro-phenyl)-3-(R)-methyl-piperazine-1-carboxylic acid tert-butyl ester
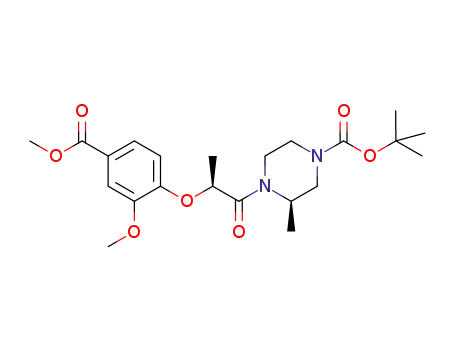
(3R)-4-[(2S)-2-(2-methoxy-4-methoxycarbonylphenoxy)propionyl]-3-methylpiperazine-1-carboxylic acid tert-butyl ester
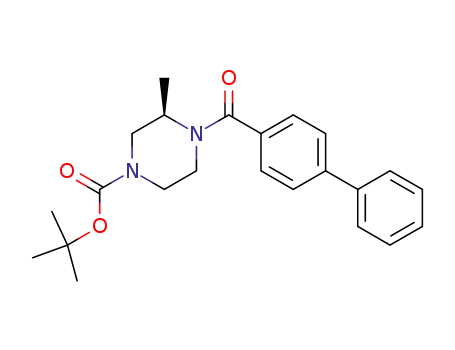
tert-butyl (3R)-4-(1,1'-biphenyl-4-ylcarbonyl)-3-methylpiperazine-1-carboxylate
CAS:56-12-2
CAS:7531-52-4
CAS:55819-71-1
CAS:147081-29-6