Your Location:Home >Products >Biochemical Engineering >71989-38-3
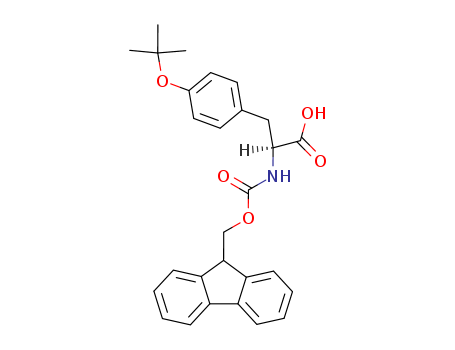

Product Details
|
Chemical Properties |
white to light yellow crystal powde |
|
Uses |
Using Fmoc-Tyr(tBu)-OH in peptide synthesis is more efficient. The use of Fmoc-Tyr(tBu)-OH also eliminates all potential for side products arising from the acylation of the tyrosine side-chain. It is used in a variety of biological and biochemical research applications, including in vivo and in vitro studies. |
|
General Description |
N-(9-Fluorenylmethoxycarbonyl)-O-tert-butyl-L-tyrosine (Fmoc-Tyr(tbu)-OH) is a self-assembled structure formed by modified single amino acids. |
InChI:InChI=1/C28H29NO5/c1-28(2,3)34-19-14-12-18(13-15-19)16-25(26(30)31)29-27(32)33-17-24-22-10-6-4-8-20(22)21-9-5-7-11-23(21)24/h4-15,24-25H,16-17H2,1-3H3,(H,29,32)(H,30,31)
Previous studies showed that the FeII/α-...
The invention relates to a method for pr...
Fmoc-O-tert-butyl-l-tyrosine (1.44 mmol, 1.2 eq) in 50 mL acetonitrile was treated with HBTU (1.4 eq), diisopropylethylamine (1.5 eq), and l-proline methyl ester (1.2 mmol, 1 eq). …
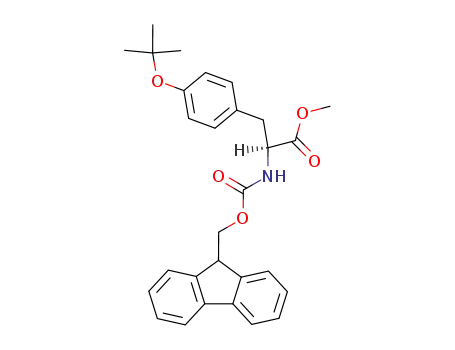
N-(9-Fluorenylmethoxycarbonyl)-O-tert-butyl-L-tyrosine Methyl Ester

Fmoc-Tyr(tBu)-OH
| Conditions | Yield |
|---|---|
|
With lithium iodide; In ethyl acetate; Reagent/catalyst; Reflux;
|
84.3% |
|
With sodium carbonate; In acetonitrile; for 15h;
|
74% |
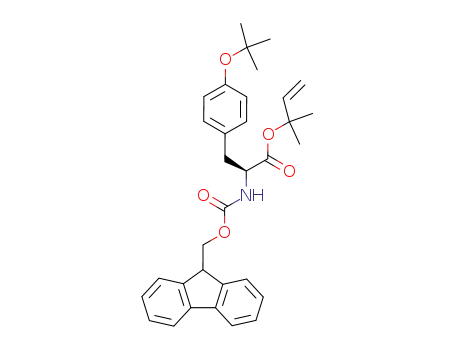
Fmoc-Tyr(t-Bu)-1,1-dimethylallyl ester


Fmoc-Tyr(tBu)-OH
| Conditions | Yield |
|---|---|
|
With 4-methyl-morpholine; tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; at 25 ℃;
|
91% |

N-(9-Fluorenylmethoxycarbonyl)-O-tert-butyl-L-tyrosine Methyl Ester
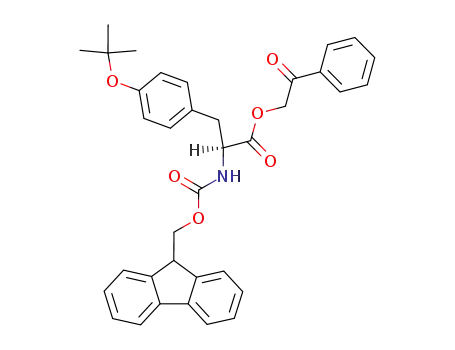
Fmoc-Tyr(but)-O-Pha

Fmoc-Tyr(t-Bu)-1,1-dimethylallyl ester
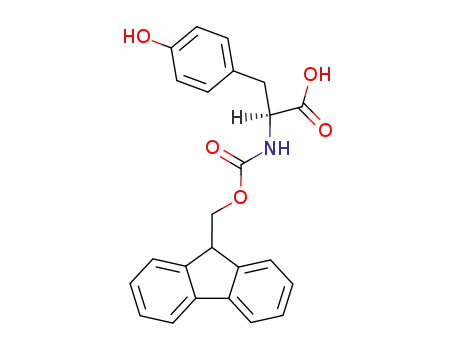
N-Fmoc-Tyr-OH
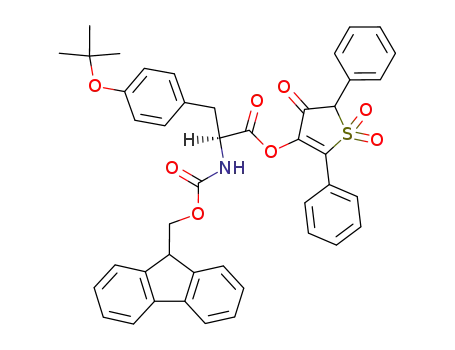
Fmoc-Tyr(tBu)-OTDO
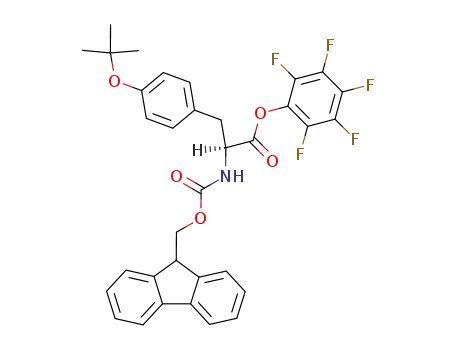
Fmoc-Tyr(tBu)-OPfp
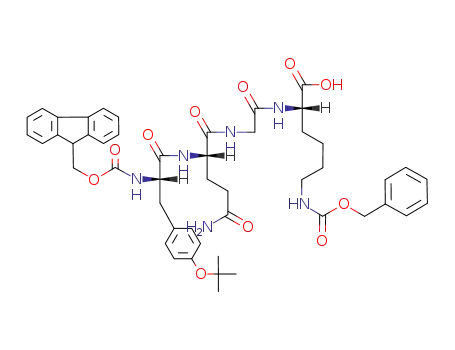
Fmoc-Tyr(t-Bu)-Gln-Gly-Lys(Z)-OH
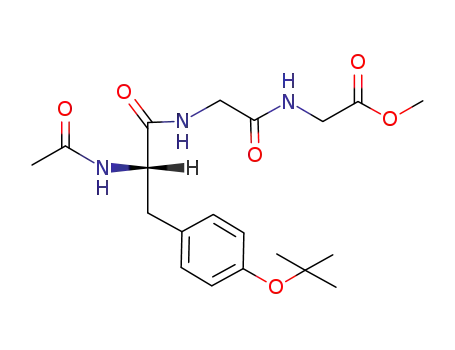
Ac-Tyr(t-Bu)-Gly-Gly-OMe
CAS:56-12-2
CAS:7531-52-4
CAS:71989-18-9
CAS:71989-33-8