Your Location:Home >Products >Biochemical Engineering >71989-33-8

Product Details
|
Chemical Properties |
white to light yellow crystal powde |
|
Uses |
Fmoc-Ser(tBu)-OH is an N-terminal protected reagent used in the peptide synthesis. It also can be used as polymer-supported. Fmoc-O-tert-Butyl-L-serine is commercially available. |
|
General Description |
Fmoc-Thr(tbu)-OH self-assembles to sphere at lower concentration which changes to dumb-bell shapes at higher concentration under room temperature conditions. |
InChI:InChI=1/C22H25NO5/c1-22(2,3)28-13-19(20(24)25)23-21(26)27-12-18-16-10-6-4-8-14(16)15-9-5-7-11-17(15)18/h4-11,18-19H,12-13H2,1-3H3,(H,23,26)(H,24,25)/p-1/t19-/m0/s1
We observed unexpectedly high levels of racemization of Fmoc-Ser(tBu)-OH during automated … We describe unexpectedly high levels of racemization of Fmoc-Ser(tBu)-OH during …
Herein we report the polymer-supported synthesis of 3,4-dihydro-2H-1,4-oxazine-3-carboxylic acid derivatives using immobilized Fmoc-Ser(tBu)-OH and Fmoc-Thr(tBu)-OH as the starting materials.
Briefly, Fmoc-O-tert-butyl-L-serine was coupled with propargylamine, giving 2, which was reacted with 2-chloroacetyl chloride upon Fmoc deprotection. The resulting intermediate 4 was …
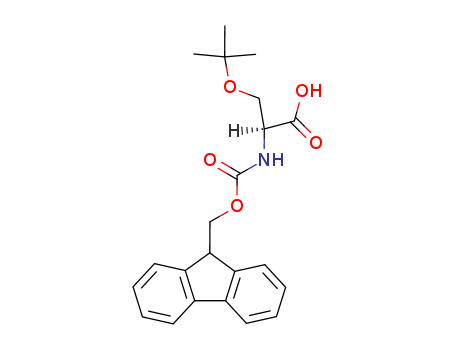
(S)-3-tert-Butoxy-2-(9H-fluoren-9-ylmethoxycarbonylamino)-propionic acid tert-butyl ester

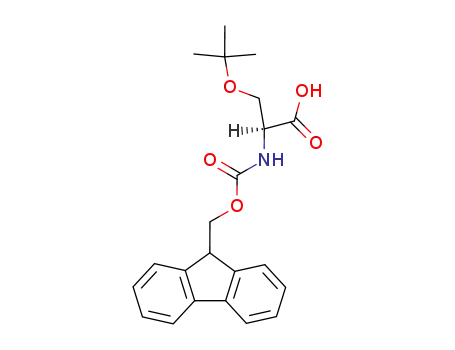
Fmoc-Ser(tBu)-OH

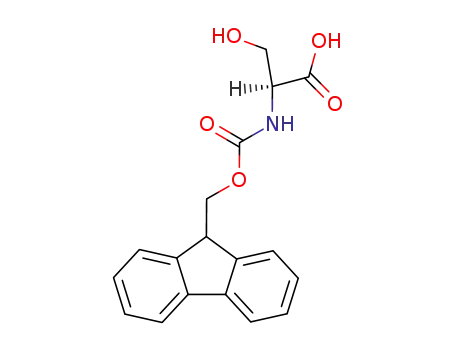
N-(9H-fluoren-9-ylmethoxycarbonyl)-L-serine
| Conditions | Yield |
|---|---|
|
With silica gel; In toluene; for 1.25h; Heating;
|
76% 8% |
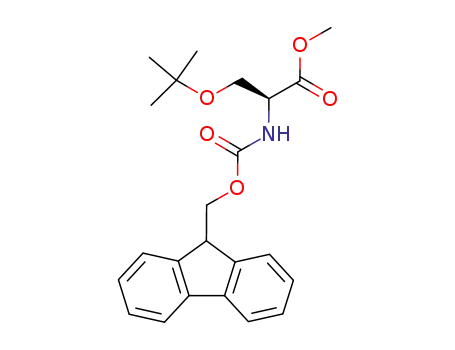
Fmoc-Ser(tBu)-OMe


Fmoc-Ser(tBu)-OH
| Conditions | Yield |
|---|---|
|
With magnesium iodide; In tetrahydrofuran; at 120 ℃; for 1h; chemoselective reaction; Inert atmosphere; Microwave irradiation; Sealed tube;
|
98% |
|
With aluminum (III) chloride; In ethyl acetate; Reflux;
|
86% |
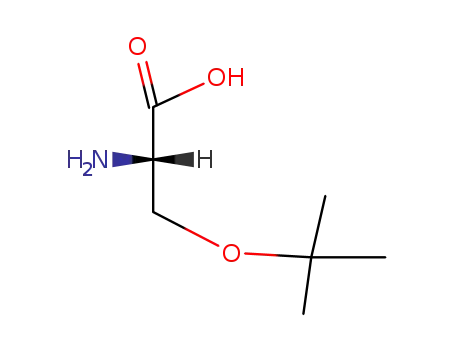
(S)-O-tert-butylserine
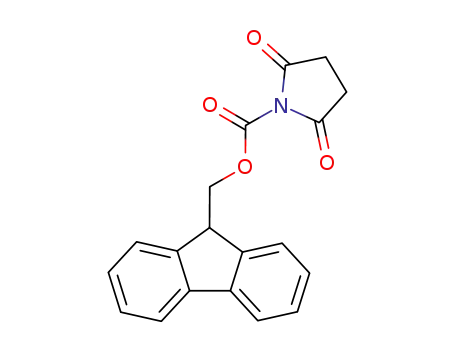
9-fluorenylmethyl N-succinimidyl carbonate
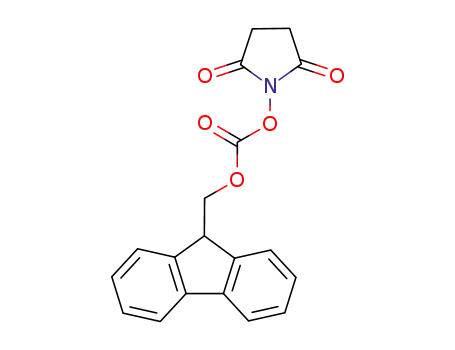
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide
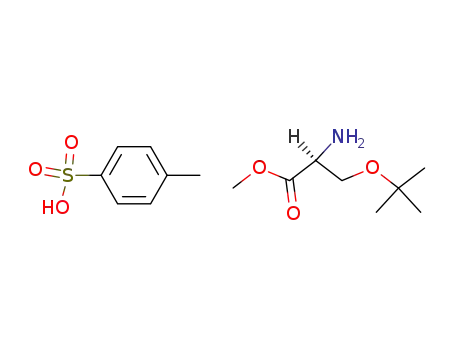
O-tert-Butyl-L-serine Methyl Ester p-Toluenesulfonate
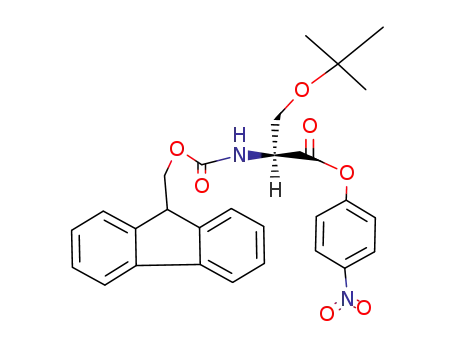
Fmoc-Ser(t-Bu)-ONp
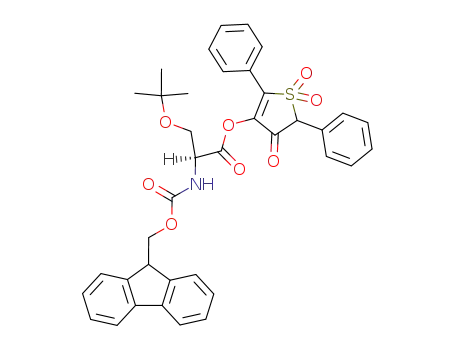
Fmoc-Ser(tBu)-OTDO
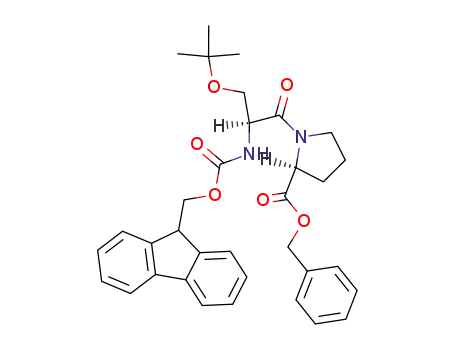
N-(9-fluorenylmethoxycarbonyl)-O-(tert-butyl)-L-seryl-L-proline benzyl ester
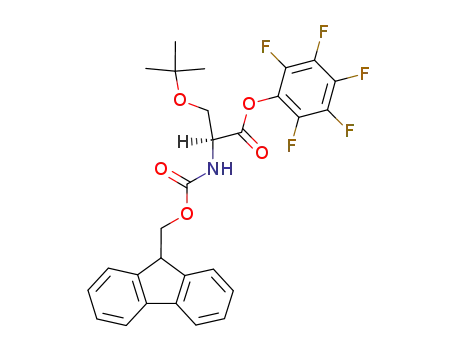
O-tert-butyl-Nα-(fluoren-9-ylmethoxycarbonyl)-L-serine pentafluorophenyl ester
CAS:56-12-2
CAS:7531-52-4
CAS:71989-38-3