Your Location:Home >Products >Biochemical Engineering >74844-91-0
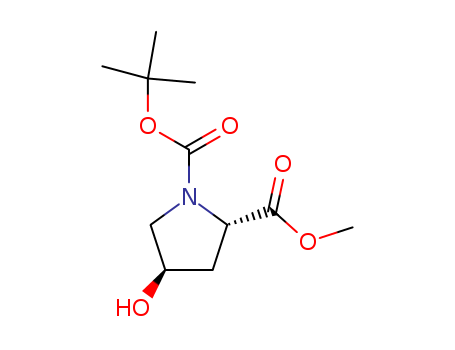

Product Details
|
Chemical Properties |
Clear Colourless Oil |
|
Uses |
N-BOC-L-Hydroxyproline methyl ester is A potential iNOS inhibitor.It is used in the synthesis of For-Met-Leu-Phe-OMe (fMLF-OMe) analogues based on Met residue replacement by 4-amino-proline scaffold. |
Isomeric SMILES: CC(C)(C)OC(=O)N1C[C@@H](C[C@H]1C(=O)OC)O
InChIKey: MZMNEDXVUJLQAF-SFYZADRCSA-N
InChI: InChI=1S/C11H19NO5/c1-11(2,3)17-10(15)12-6-7(13)5-8(12)9(14)16-4/h7-8,13H,5-6H2,1-4H3/t7-,8+/m1/s1
At this stage, the addition of either the cis iodide 8 or the trans tosylate 9 (Scheme 4), prepared as described above from N-Boc-l-hydroxyproline methyl ester, followed by slow cooling of …
Azanucleosides, the sugar-modified nucle...
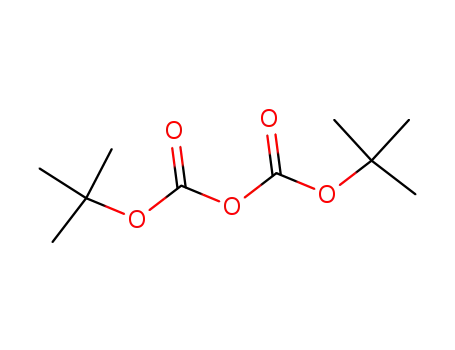
di-tert-butyl dicarbonate

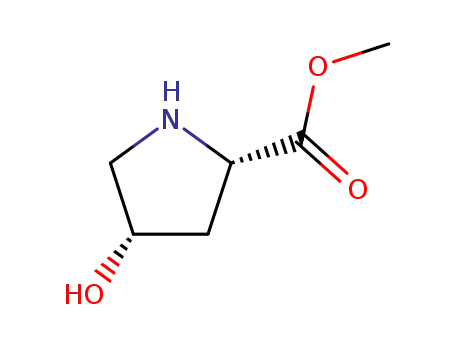
cis-L-hydroxyproline methyl ester

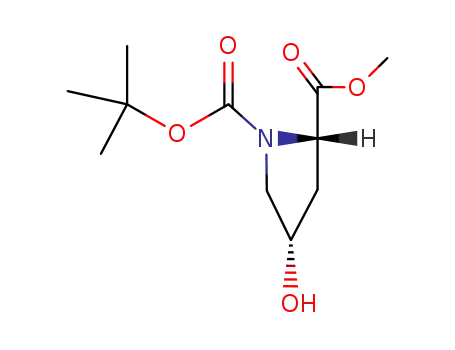
(2S,4S)-N-tert-butoxycarbonyl-4-hydroxyproline methyl ester
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In methanol; at 20 ℃; Inert atmosphere;
|
90% |
|
With triethylamine; In tetrahydrofuran; at 0 - 20 ℃; for 16.5h;
|
65% |
|
With triethylamine; In dichloromethane; Yield given; Ambient temperature;
|
|
|
With triethylamine;
|
|
|
With sodium hydrogencarbonate; In chloroform; water; at 20 ℃; for 2h;
|
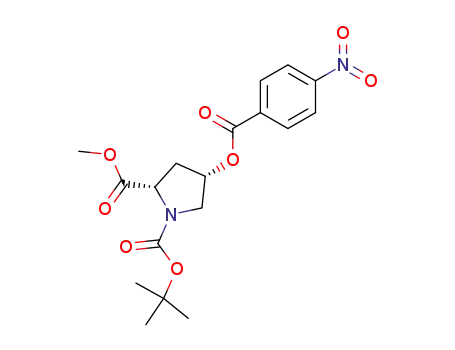
Boc-(2S,4S)-p-nitrobenzoate-4-hydroxyproline methyl ester


(2S,4S)-N-tert-butoxycarbonyl-4-hydroxyproline methyl ester
| Conditions | Yield |
|---|---|
|
With sodium methylate; In methanol;
|
95% |
|
With methanol; sodium azide; at 40 ℃; for 4h;
|
85% |
|
With sodium azide; In methanol; at 40 ℃; for 40h; Inert atmosphere;
|
73% |
|
With sodium azide; In acetone; for 14h; Reflux;
|
65% |
|
Boc-(2S,4S)-p-nitrobenzoate-4-hydroxyproline methyl ester; With sodium azide; In acetone; for 14h; Reflux;
With water; In ethyl acetate;
|
65% |
|
Boc-(2S,4S)-p-nitrobenzoate-4-hydroxyproline methyl ester; With methanol; potassium hydroxide; at 0 ℃; for 0.5h; Cooling with ice bath; Inert atmosphere;
With hydrogenchloride; In methanol; water; Inert atmosphere;
|

diazomethane
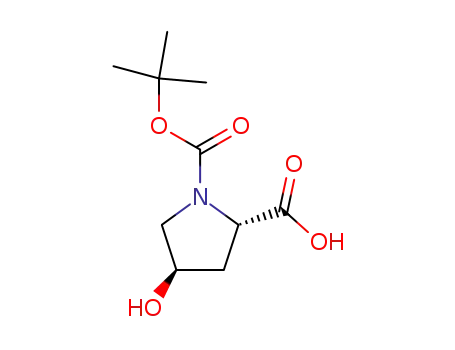
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid

di-tert-butyl dicarbonate
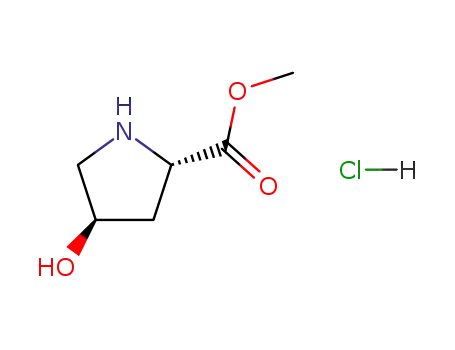
L-4-hydroxyproline methyl ester hydrochloride
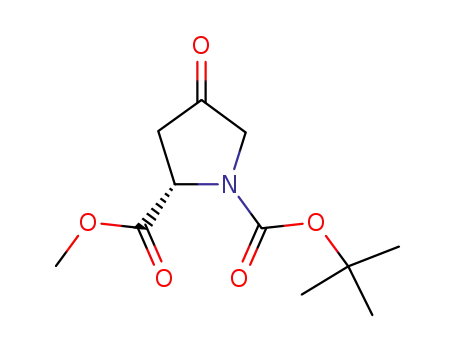
(S)-1-tert-butyl 2-methyl 4-oxopyrrolidine-1,2-dicarboxylate

(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid
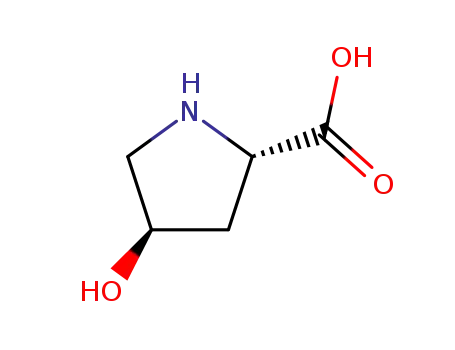
4R-4-hydroxyproline
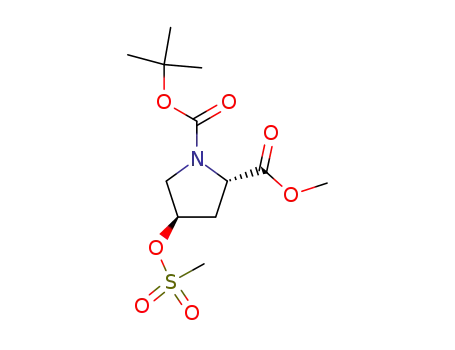
(2S,4R)-4-(methylsulfonyloxy)pyrrolidine-1,2-dicarboxylic acid 1-tert-butylester-2-methylester
CAS:56-12-2
CAS:7531-52-4
CAS:102195-80-2
CAS:3303-84-2