Your Location:Home >Products >Biochemical Engineering >3303-84-2
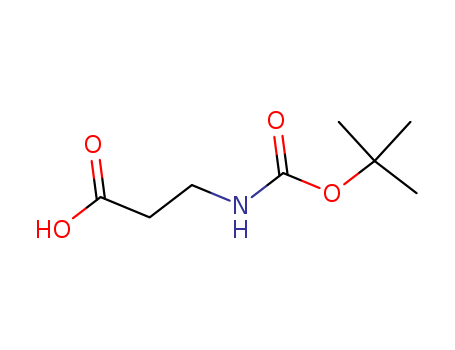

Product Details
|
Description |
Boc-beta-Ala-OH is an alkane chain with terminal carboxlic acid and Boc-protected amino groups. |
|
Chemical Properties |
White solid |
|
Uses |
Boc-beta-alanine can be used as a PROTAC linker in the synthesis of PROTACs. |
InChI:InChI=1/C8H15NO4/c1-8(2,3)13-7(12)9-5-4-6(10)11/h4-5H2,1-3H3,(H,9,12)(H,10,11)/p-1
… study involved the condensation of BOC-beta-alanine with the bifunctional alkylating agent melphalan. The melphalan and BOC-beta-alanine were purchased from Sigma Chemical …
Then Masamune-Claisen beta keto ester 2 was assembled in one pot through the reaction of N- t Boc beta-alanine with Meldrum’s acid and further the intermediate formed in situ was …
Alternatively, the Boc-β-Fr-OMe dimer (22) can be synthesized from 1 by coupling to the symmetrical anhydride of Boc-beta-alanine in DMF, DIEA, and DMAP. The anhydride was pre-…
di-tert-butyl dicarbonate

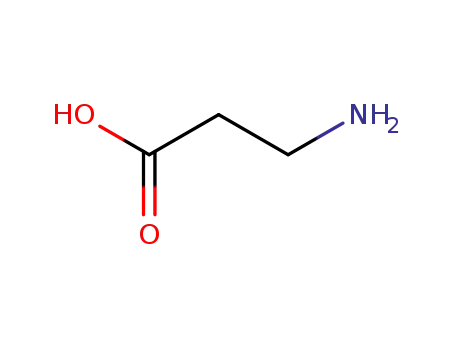
3-amino propanoic acid

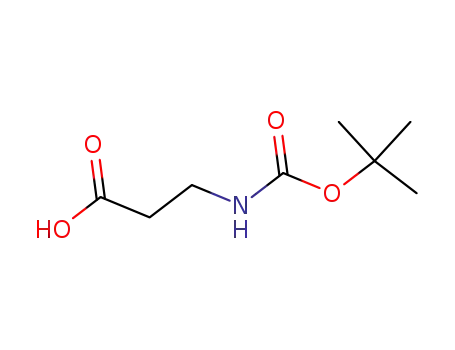
3-(tert-butyloxycarbonylamino)propionic acid
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; tert-butyl alcohol; at 20 ℃;
|
100% |
|
With sodium hydroxide; In 1,4-dioxane; water; at 0 ℃;
|
100% |
|
With sodium hydroxide; In tetrahydrofuran; at 20 ℃; for 16h;
|
100% |
|
With sodium hydroxide; In methanol; at 0 - 20 ℃;
|
99% |
|
With potassium carbonate; In 1,4-dioxane; water;
|
98% |
|
With sodium hydroxide; In 1,4-dioxane; water; at 0 - 25 ℃; for 0.5h;
|
98% |
|
With sodium hydroxide; In water; tert-butyl alcohol; at 20 ℃; for 16h;
|
97% |
|
With sodium hydroxide; In 1,4-dioxane; water; at 20 ℃;
|
97% |
|
With potassium carbonate; In 1,4-dioxane; water; at 20 ℃; for 24h;
|
96% |
|
With sodium hydroxide; In tetrahydrofuran; at 0 - 20 ℃;
|
95% |
|
With sodium hydroxide; In methanol; chloroform; at 20 ℃; for 3h;
|
94.17% |
|
With sodium hydroxide; at 20 ℃; for 20h;
|
94% |
|
With sodium hydroxide; at 20 ℃; for 20h;
|
94% |
|
With sodium hydroxide; In 1,4-dioxane; at 20 ℃; for 6h;
|
92% |
|
With sodium hydroxide; In 1,4-dioxane; at 20 ℃;
|
91% |
|
With sodium hydroxide; In water; tert-butyl alcohol; for 12h; Ambient temperature;
|
90% |
|
With sodium hydroxide; at 0 - 20 ℃; for 3h;
|
90% |
|
With sodium hydroxide; In 1,4-dioxane; 0 deg C to room temp.,;
|
88% |
|
With sodium hydroxide; In 1,4-dioxane; water; at 30 ℃; for 6h;
|
88.85% |
|
With sodium hydroxide; In 1,4-dioxane; water; at 0 - 20 ℃;
|
88% |
|
With sodium hydroxide; In 1,4-dioxane; water; at 20 ℃; for 0.5h;
|
87% |
|
With sodium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃;
|
87% |
|
With sodium hydroxide; In water; tert-butyl alcohol; at 20 - 40 ℃; for 16h;
|
86% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 0 - 20 ℃; for 4h;
|
85% |
|
3-amino propanoic acid; With sodium hydroxide; In 1,4-dioxane; water; at 0 ℃; for 0.0833333h;
di-tert-butyl dicarbonate; In 1,4-dioxane; water; at 0 - 20 ℃;
|
82% |
|
With sodium hydroxide; Ambient temperature;
|
80% |
|
With sodium hydroxide; In 1,4-dioxane; at 0 - 30 ℃; for 16h;
|
80% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 5 - 20 ℃; for 12h;
|
80% |
|
With sodium hydroxide; In dichloromethane; at 0 - 20 ℃; for 12h; Inert atmosphere;
|
80% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 0 - 20 ℃; for 12h;
|
79% |
|
With sodium hydroxide; In tetrahydrofuran; at 20 ℃; for 16h;
|
76% |
|
With sodium hydroxide; tert-butyl alcohol; In water; at 30 ℃; for 18h;
|
73% |
|
With dmap; In dichloromethane; at 0 - 20 ℃;
|
70% |
|
With dmap; In dichloromethane; at 0 - 20 ℃; Inert atmosphere;
|
70% |
|
With potassium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 12h;
|
68% |
|
With sodium hydroxide; In 1,4-dioxane; at 0 - 20 ℃; for 16h; Inert atmosphere;
|
56% |
|
With sodium hydroxide; In tetrahydrofuran; water; at 0 - 20 ℃; for 16h;
|
51% |
|
With sodium hydroxide; In tetrahydrofuran; at 0 - 20 ℃; for 16h;
|
51% |
|
With sodium hydroxide; In isopropyl alcohol; at 0 - 20 ℃; for 2h;
|
50% |
|
With sodium hydroxide; In water; tert-butyl alcohol; at 20 ℃; for 16h; Inert atmosphere;
|
8% |
|
With potassium carbonate; In 1,4-dioxane; water; for 2.5h; Ambient temperature;
|
|
|
With sodium hydroxide; In tert-butyl alcohol; for 12h;
|
|
|
With sodium hydroxide; In 1,4-dioxane; water; at 0 - 20 ℃;
|
|
|
With sodium hydroxide;
|
|
|
In sodium hydrogencarbonate; ethyl acetate; acetonitrile;
|
3.71 g (98%) |
|
With sodium hydroxide; In water;
|
|
|
With sodium hydrogencarbonate; In 1,4-dioxane; water; Cooling with ice;
|
|
|
di-tert-butyl dicarbonate; 3-amino propanoic acid; With potassium hydroxide; In 1,4-dioxane; water; at 20 ℃; for 12h;
With sodium hydrogen sulfate; In water; ethyl acetate;
|
|
|
With potassium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃;
|
|
|
With sodium hydroxide; In 1,4-dioxane; water;
|
|
|
With water; sodium hydroxide; In tert-butyl alcohol;
|
|
|
di-tert-butyl dicarbonate; 3-amino propanoic acid; With sodium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 6h; pH=9 - 11;
With citric acid; In water; pH=4 - 5;
|
|
|
With sodium hydroxide; In tert-butyl alcohol; at 20 ℃;
|
|
|
With sodium hydroxide; at 20 ℃; for 3h;
|
|
|
With water; sodium hydroxide; In tetrahydrofuran; at 0 - 20 ℃; for 0.5h;
|
|
|
With sodium hydroxide; In 1,4-dioxane; at 20 ℃;
|
|
|
With potassium hydroxide; In 1,4-dioxane; water; at 25 ℃; for 12h;
|
|
|
With sodium hydroxide; In tetrahydrofuran; at 0 - 20 ℃; for 0.5h;
|
|
|
With sodium hydroxide; In aq. phosphate buffer;
|
1.89 g |
|
di-tert-butyl dicarbonate; 3-amino propanoic acid; With sodium hydroxide; In water; at 20 ℃; for 12h;
With hydrogenchloride; In water;
|
|
|
With sodium hydroxide; In water; tert-butyl alcohol; at 20 ℃; for 16h;
|
|
|
With sodium carbonate; In tetrahydrofuran; water; Inert atmosphere;
|
|
|
With sodium hydroxide; In isopropyl alcohol; at 0 - 20 ℃; for 2h;
|
|
|
With sodium hydroxide; In 1,4-dioxane; water; at 20 ℃; for 18h;
|
1.26 g |
|
With sodium hydroxide; In tetrahydrofuran; water; at 0 - 20 ℃; for 16.0833h; Inert atmosphere;
|
|
|
With sodium carbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 25h;
|
|
|
With sodium hydroxide; In water; tert-butyl alcohol; at 0 - 20 ℃;
|
|
|
With sodium hydroxide; In tert-butyl alcohol; at 20 ℃;
|
|
|
With sodium hydroxide; In 1,4-dioxane; water; at 20 ℃; for 8h;
|
|
|
di-tert-butyl dicarbonate; 3-amino propanoic acid; With sodium hydroxide; for 0.333333h; Cooling with ice;
In water; at 20 ℃; for 14h; Further stages;
|
3.39 g |
|
With sodium hydroxide; In 1,4-dioxane; water; at 0 - 20 ℃; for 3h;
|
|
|
In tetrahydrofuran; water; for 18h; Reflux;
|
|
|
With triethylamine; In N,N-dimethyl-formamide; at 20 - 40 ℃; Sonication;
|
|
|
With sodium hydroxide; In 1,4-dioxane; water; at 20 ℃; for 3h; Inert atmosphere;
|
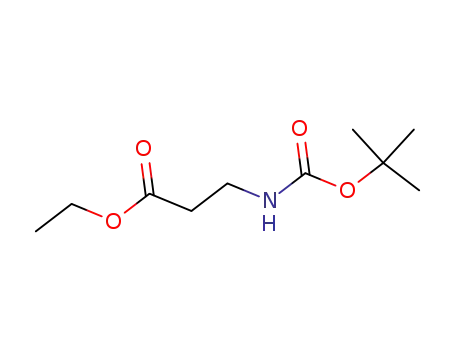
3-tert-butoxycarbonylamino-propionic acid ethyl ester


3-(tert-butyloxycarbonylamino)propionic acid
| Conditions | Yield |
|---|---|
|
With water; lithium hydroxide; In ethanol; at 20 ℃; for 5h;
|
90% |
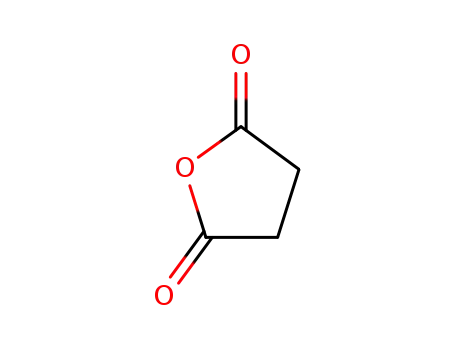
succinic acid anhydride
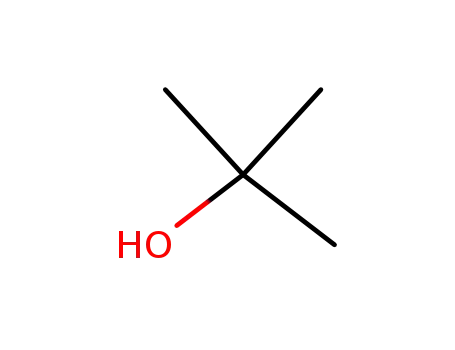
tert-butyl alcohol
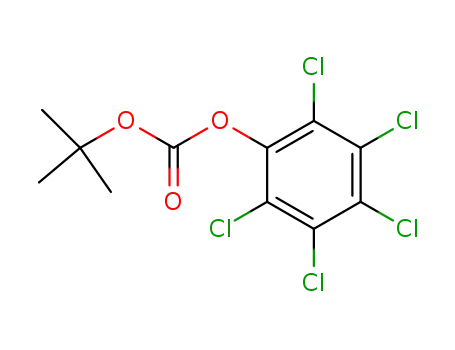
t-butylpentachlorophenyl carbonate
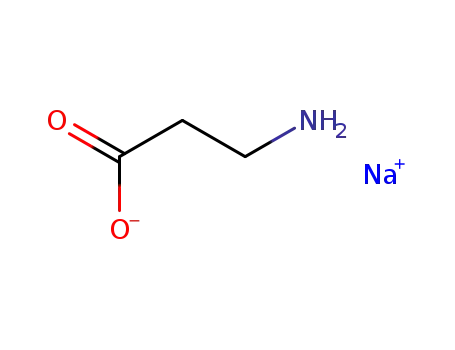
sodium β-alaninate
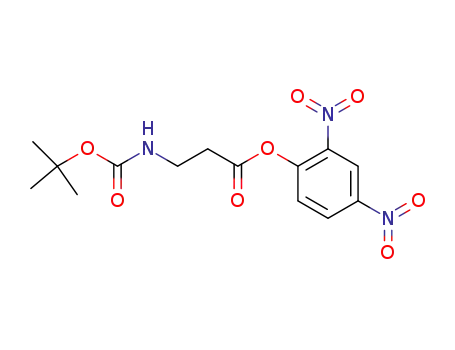
2,4-Dinitrophenylester von BOC-β-Alanin
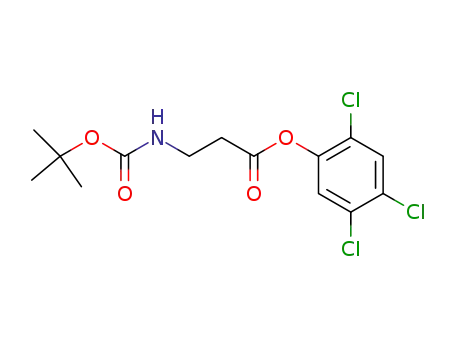
2,4,5-trichlorophenyl 3-<(tert-butyloxycarbonyl)amino>propanoate
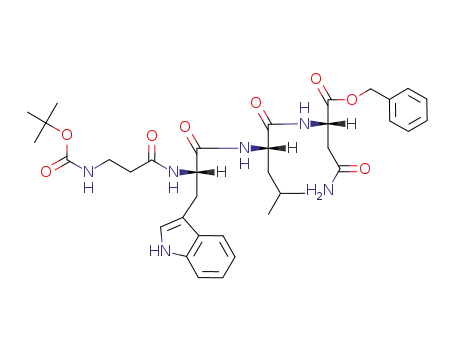
Boc-β-Ala-L-Trp-L-Leu-L-Asn-OBzl
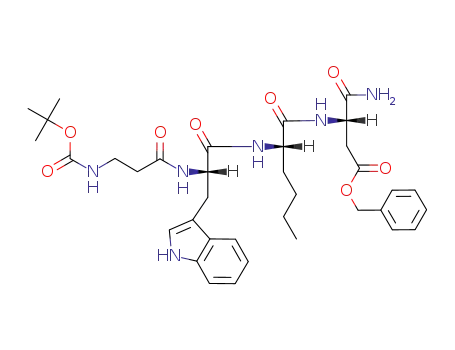
Boc-β-Ala-L-Trp-L-Nle-L-Asp(Bzl)-NH2
CAS:56-12-2
CAS:7531-52-4
CAS:74844-91-0
CAS:13734-36-6