Your Location:Home >Products >Biochemical Engineering >102195-80-2
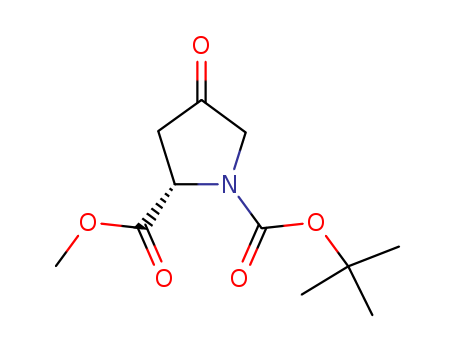

Product Details
|
Chemical Properties |
Yellow Solid |
|
Uses |
N-Boc-4-oxo-L-proline methyl ester is a compound useful in organic synthesis. |
Isomeric SMILES: CC(C)(C)OC(=O)N1CC(=O)C[C@H]1C(=O)OC
InChIKey: UPBHYYJZVWZCOZ-QMMMGPOBSA-N
InChI: InChI=1S/C11H17NO5/c1-11(2,3)17-10(15)12-6-7(13)5-8(12)9(14)16-4/h8H,5-6H2,1-4H3/t8-/m0/s1
N-Boc-4-oxo-l-proline (4) is a suitable intermediate for the synthesis of fluorinated prolines 7 and 9. N-Boc-4-oxo-l-proline (4) is … trioxide and pyridine to give N-Boc-4-oxo-l-proline (4). …
… or enolate equivalent of N-Boc-4-oxo-l-proline benzyl ester 1, which is … from readily available N-Boc-4-oxo-l-proline benzyl ester. … 4-oxo-l-proline methyl ester. By means of computational …
trimethylsilyl diazomethane

(28)-1-(tert-butoxycarbonyl)-4-oxo-2-pyrrolidinecarboxylic acid

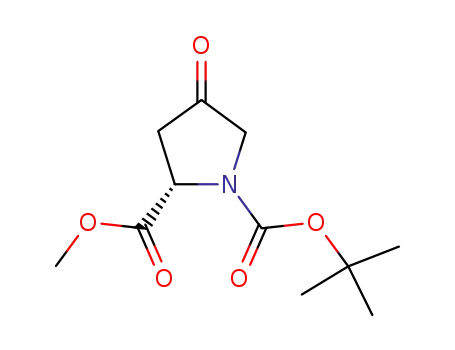
(S)-1-tert-butyl 2-methyl 4-oxopyrrolidine-1,2-dicarboxylate
| Conditions | Yield |
|---|---|
|
In methanol; toluene;
|
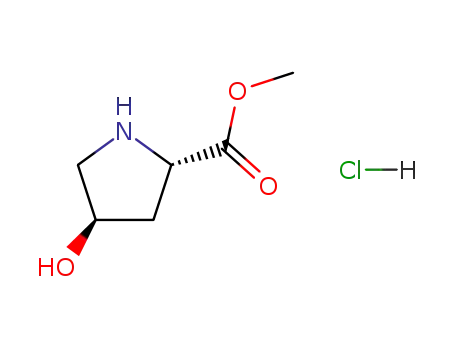
L-4-hydroxyproline methyl ester hydrochloride


(S)-1-tert-butyl 2-methyl 4-oxopyrrolidine-1,2-dicarboxylate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 17.7 g / triethylamine / dimethylformamide / 1.5 h / 55 °C
2: 90 percent / N-methylmorpholine-N-oxide; 4 Angstroem molecular sieves; tetrapropylammonium perruthenate / CH2Cl2 / 3.5 h / 35 °C
With tetrapropylammonium perruthennate; 4 A molecular sieve; 4-methylmorpholine N-oxide; triethylamine; In dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: 100 percent / aq. NaOH / tetrahydrofuran / 20 °C
2: trichloroisocyanuric acid; TEMPO / CH2Cl2
With 2,2,6,6-tetramethyl-piperidine-N-oxyl; sodium hydroxide; trichloroisocyanuric acid; In tetrahydrofuran; dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: 95 percent / Et3N / CH2Cl2 / 2 h / 0 - 20 °C
2: 93 percent / (COCl)2; Et3N; DMSO / CH2Cl2 / -78 °C
With oxalyl dichloride; dimethyl sulfoxide; triethylamine; In dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: 89 percent / Et3N / dioxane; H2O / 2 h / 20 °C
2: 50 percent / PDC / dimethylformamide / 8 h / 20 °C
With dipyridinium dichromate; triethylamine; In 1,4-dioxane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: 95 percent / Et3N / CH2Cl2 / Ambient temperature
2: 81 percent / PDC, 3 Angstroem molecular sieves / CH2Cl2 / Ambient temperature
With dipyridinium dichromate; 3 A molecular sieve; triethylamine; In dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: triethylamine
2: sodium periodate; ruthenium trichloride
With ruthenium trichloride; sodium periodate; triethylamine;
|
|
|
Multi-step reaction with 2 steps
1: triethylamine / dichloromethane / 4 h / 0 - 20 °C
2: pyridinium chlorochromate / dichloromethane / 20 °C
With triethylamine; pyridinium chlorochromate; In dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: triethylamine / dichloromethane / 20 °C
2: Dess-Martin periodane / dichloromethane / 4 h / 0 - 20 °C
With Dess-Martin periodane; triethylamine; In dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1.1: sodium hydrogencarbonate / methanol
2.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 2 h / -78 °C
2.2: -78 - 20 °C
With oxalyl dichloride; sodium hydrogencarbonate; dimethyl sulfoxide; In methanol; dichloromethane; 2.1: |Swern Oxidation / 2.2: |Swern Oxidation;
|
|
|
Multi-step reaction with 2 steps
1.1: sodium hydrogencarbonate / methanol; water / 8 h
2.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 2 h / -78 °C
2.2: -78 - 20 °C
With oxalyl dichloride; sodium hydrogencarbonate; dimethyl sulfoxide; In methanol; dichloromethane; water; 2.1: |Swern Oxidation;
|
|
|
Multi-step reaction with 2 steps
1: sodium hydrogencarbonate / methanol / 8 h
2: dimethyl sulfoxide; oxalyl dichloride / dichloromethane / 2 h / -78 °C
With oxalyl dichloride; sodium hydrogencarbonate; dimethyl sulfoxide; In methanol; dichloromethane; 2: |Swern Oxidation;
|
|
|
Multi-step reaction with 2 steps
1: triethylamine / acetonitrile / 0 - 20 °C / Inert atmosphere
2: trichloroisocyanuric acid; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical / dichloromethane / 0.25 h / 0 - 20 °C
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; trichloroisocyanuric acid; triethylamine; In dichloromethane; acetonitrile;
|
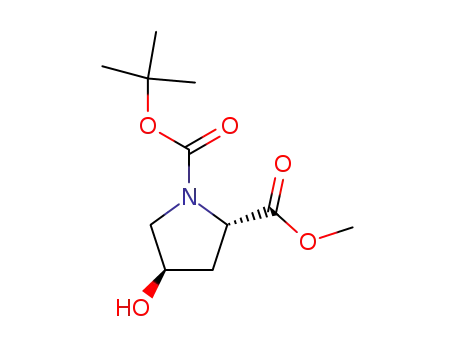
1-tert-butyl 2-methyl (2S,4R)-4-hydroxy-1,2-pyrrolidinedicarboxylate
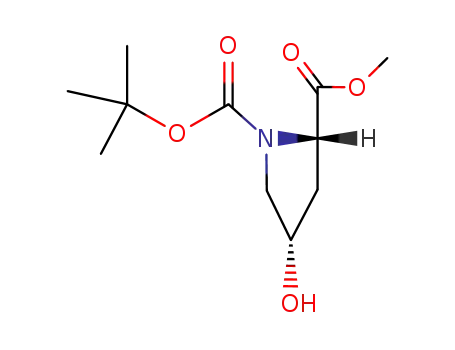
(2S,4S)-N-tert-butoxycarbonyl-4-hydroxyproline methyl ester
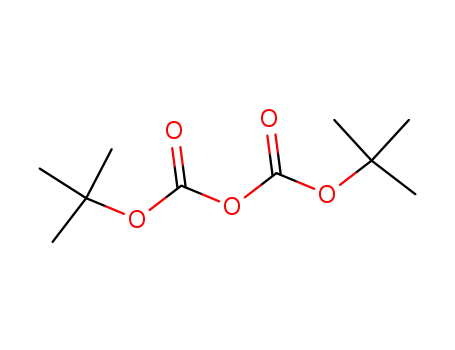
di-tert-butyl dicarbonate
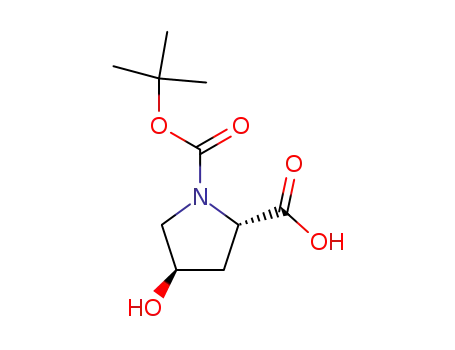
(2S,4R)-4-hydroxy-1-[(2-methylpropan-2-yl)oxycarbonyl]pyrrolidine-2-carboxylic acid
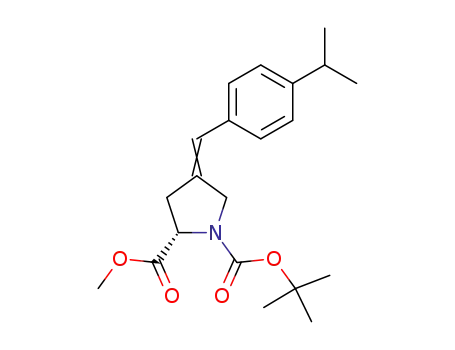
(S)-4-[1-(4-Isopropyl-phenyl)-meth-(E)-ylidene]-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester 2-methyl ester
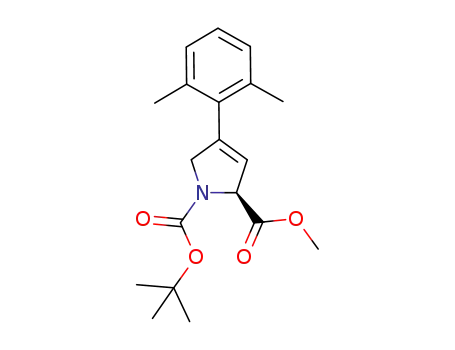
1-tert-butyl 2-methyl (2S)-4-(2,6-dimethylphenyl)-2,5-dihydro-1H-pyrrole-1,2-dicarboxylate
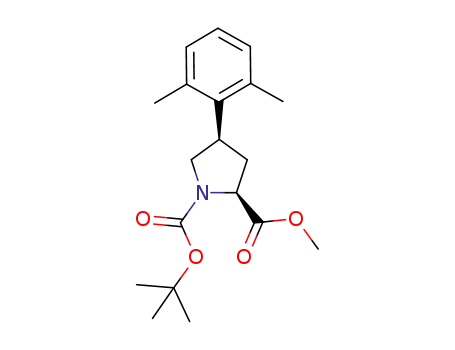
1-tert-butyl 2-methyl (2S,4R)-4-(2,6-dimethylphenyl)-1,2-pyrrolidinedicarboxylate
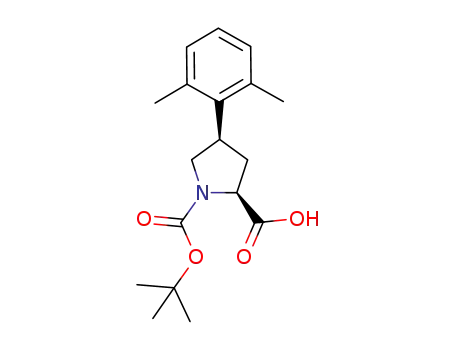
(2S,4R)-1-(tert-butoxycarbonyl)-4-(2,6-dimethylphenyl)-2-pyrrolidinecarboxylic acid
CAS:56-12-2
CAS:7531-52-4
CAS:59279-60-6
CAS:74844-91-0