Your Location:Home >Products >Biochemical Engineering >59279-60-6

Product Details
|
Uses |
N-Boc-L-glutamic Acid 1,5-Dimethyl Ester is a useful research chemical. It can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development and chemical production processes. N-Boc-L-Glutamic Acid dimethyl ester is also used in the design of antiviral drug candidates targeting the SARS-CoV-2 main protease. |
| European Community (EC) Number | 858-937-3 |
| XLogP3-AA | 1.1 |
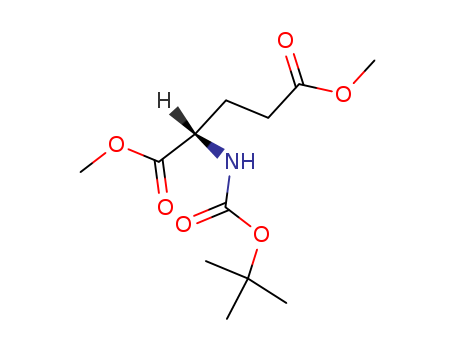
Isomeric SMILES: CC(C)(C)OC(=O)N[C@@H](CCC(=O)OC)C(=O)OC
InChIKey: QNSPKWUAZQIIGZ-QMMMGPOBSA-N
InChI: InChI=1S/C12H21NO6/c1-12(2,3)19-11(16)13-8(10(15)18-5)6-7-9(14)17-4/h8H,6-7H2,1-5H3,(H,13,16)/t8-/m0/s1
An efficient synthetic route to a key intermediate for the preparation of the rhinovirus protease inhibitor AG7088 has been developed employing a key asymmetric dianionic cyanomethylation of N-Boc-l-(+)-glutamic acid dimethyl ester. This methodology enables the preparation of this compound in kilogram quantities with an overall yield of 30%.
The invention belongs to the technical f...
![dimethyl (2S)-2-{N-[(tert-butoxy)carbonyl]benzyloxycarbonylamino}pentane-1,5-dioate](/upload/2023/6/203ec2a3-6ea0-4da4-b909-9687ef47fbad.png)
dimethyl (2S)-2-{N-[(tert-butoxy)carbonyl]benzyloxycarbonylamino}pentane-1,5-dioate

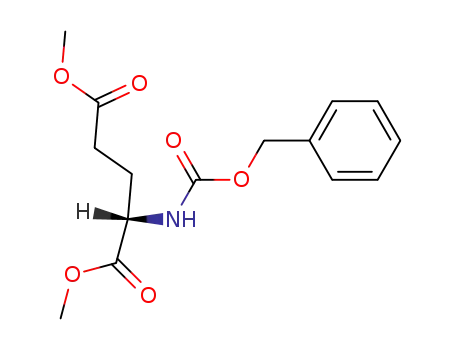
dimethyl (S)-N-(benzyloxycarbonyl)glutamate

![dimethyl (2S)-2-[(tert-butoxycarbonyl)amino]pentanedioate](/upload/2023/6/96b4c671-4e71-4af5-ad39-5484e4c6c865.png)
dimethyl (2S)-2-[(tert-butoxycarbonyl)amino]pentanedioate
| Conditions | Yield |
|---|---|
|
With lithium bromide; In acetonitrile; at 65 ℃; for 10h;
|
|
|
With lithium bromide; In acetonitrile; at 65 ℃; for 10h; Title compound not separated from byproducts.;
|
di-tert-butyl dicarbonate

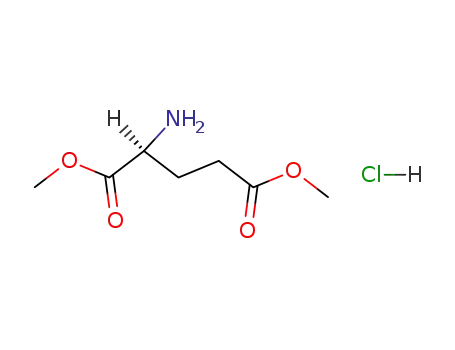
L-glutamic dimethyl ester hydrochloride

![dimethyl (2S)-2-[(tert-butoxycarbonyl)amino]pentanedioate](/upload/2023/6/96b4c671-4e71-4af5-ad39-5484e4c6c865.png)
dimethyl (2S)-2-[(tert-butoxycarbonyl)amino]pentanedioate
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In tetrahydrofuran; water; at 20 ℃; for 16h; Inert atmosphere;
|
100% |
|
With iodine; at 20 ℃; for 1h;
|
88% |
|
With zirconium(IV) chloride; In acetonitrile; at 20 ℃; for 0.166667h;
|
80% |
|
With triethylamine; dmap; In dichloromethane; at 0 - 20 ℃;
|
68% |
|
With triethylamine; In methanol; for 18h;
|
|
|
With sodium carbonate; In water; ethyl acetate; at 20 ℃; for 18h;
|
|
|
With dmap; triethylamine; In 1,4-dioxane; water;
|
|
|
With triethylamine; In methanol; for 6h; Cooling with ice;
|
|
|
With triethylamine; In dichloromethane; at 0 - 20 ℃; for 10.25h; Inert atmosphere;
|
|
|
With sodium hydroxide; In water; ethyl acetate; for 12h; pH=9 - 10; Large scale;
|
843 g |

diazomethane
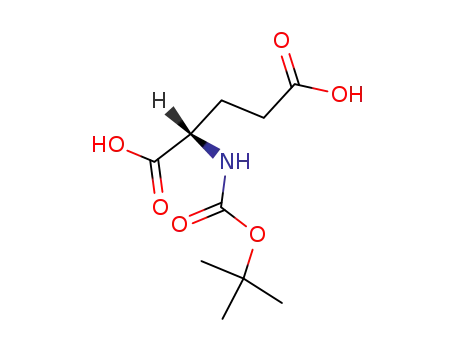
Boc-Glu
methanol
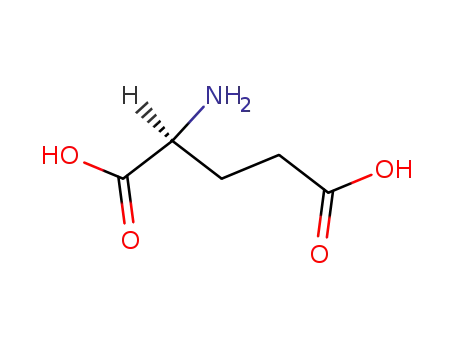
L-glutamic acid
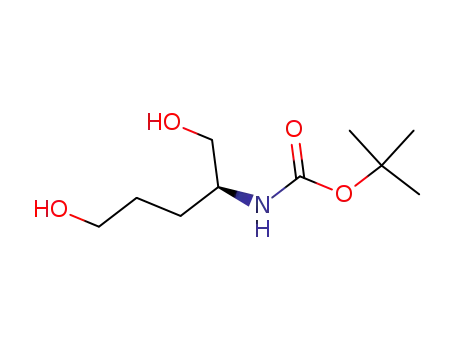
[(1S)-4-hydroxy-1-(hydroxymethyl)butyl]carbamic acid tert-butyl ester
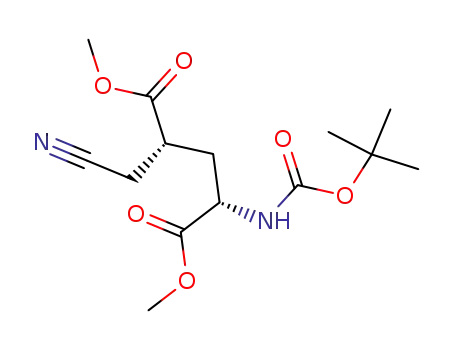
(2S,4S)-2-tert-butoxycarbonylamino-4-cyanomethyl-pentadioic acid dimethyl ester
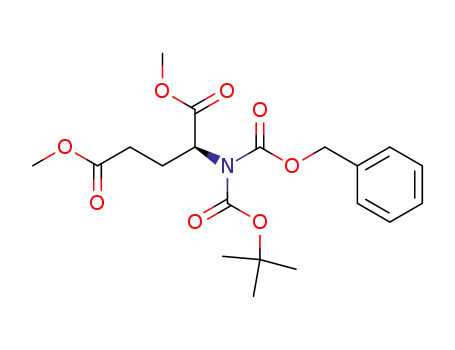
dimethyl (2S)-2-{N-[(tert-butoxy)carbonyl]benzyloxycarbonylamino}pentane-1,5-dioate
C31H41N3O5
CAS:56-12-2
CAS:7531-52-4
CAS:45214-91-3
CAS:102195-80-2