Your Location:Home >Products >Biochemical Engineering >45214-91-3
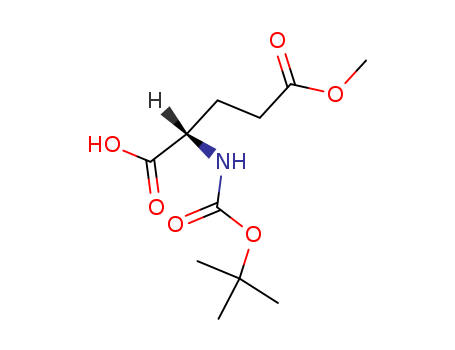

Product Details
|
Uses |
N-Boc-L-glutamic Acid 5-Methyl Ester is an intermediate used in the synthesis of Isodesmosine Chloride Hydrate (Synthetic) (I815051), which is a component of elastin and also it is extremely hygroscopic and must be stored over a desiccant such as silica gel. |
Isomeric SMILES: CC(C)(C)OC(=O)N[C@@H](CCC(=O)OC)C(=O)O
InChIKey: OHYMUFVCRVPMEY-ZETCQYMHSA-N
InChI: InChI=1S/C11H19NO6/c1-11(2,3)18-10(16)12-7(9(14)15)5-6-8(13)17-4/h7H,5-6H2,1-4H3,(H,12,16)(H,14,15)/t7-/m0/s1
… from racemic glutamic acid 5-methyl ester exhibited two … derived from L-glutamic acid 5methyl ester showed only a singlet at … iV-Boc-L-glutamic dithiol ester 67 was acylated with the acid …
The new 1H-furo[3,2-b]imidazo[4,5-d]pyri...
We describe here an efficient synthesis ...
(S)-(-)-1-tert-butyl-5-methyl-<(2-tert-butoxycarbonyl)amino>pentanedioate

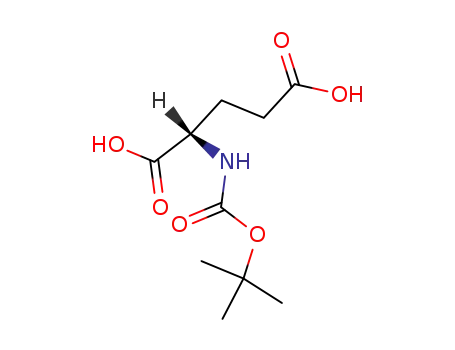
Boc-Glu

![(2S)-2-[(tert-butoxy)carbonylamino]-4-(methoxycarbonyl)butanoic acid](/upload/2023/6/1a3dd44f-052e-407d-a8fb-984042ccd1b5.png)
(2S)-2-[(tert-butoxy)carbonylamino]-4-(methoxycarbonyl)butanoic acid

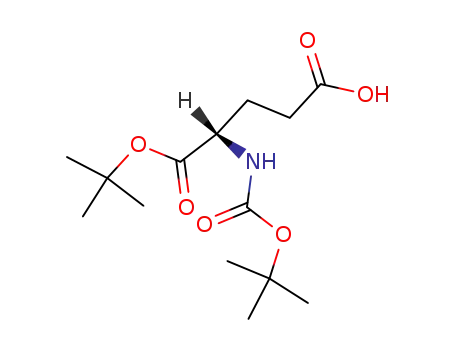
N-tert-butoxycarbonyl glutamic acid tert-butyl ester
| Conditions | Yield |
|---|---|
|
With Candida antarctica lipase; phosphate buffer; In methanol; hexane; at 37 ℃; for 48h;
|
20% 31% 19% |
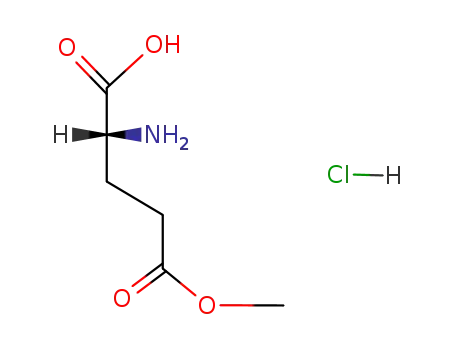
D-glutamic acid-5-methyl ester; hydrochloride

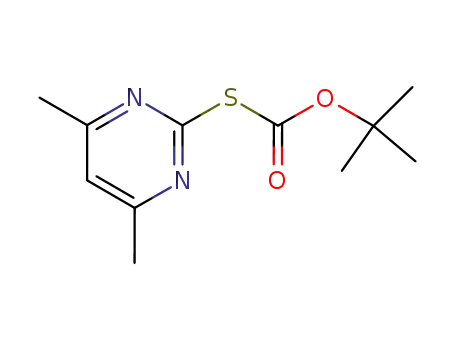
S-tert-butoxycarbonyl-4,6-dimethyl-2-mercaptopyrimidine

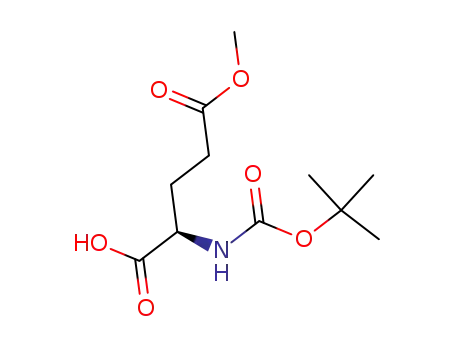
N-α-(tert-butoxycarbonyl)-D-glutamic acid γ-methyl ester
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; triethanolamine; triethylamine; In water; N,N-dimethyl-formamide;
|
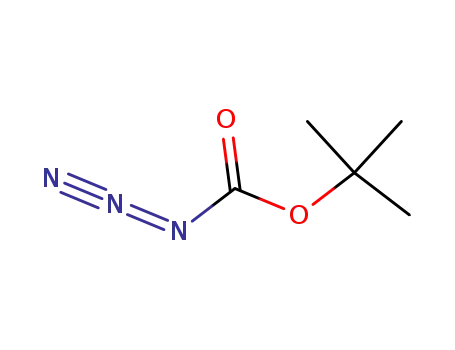
N-(tert-butyloxycarbonyl) azide
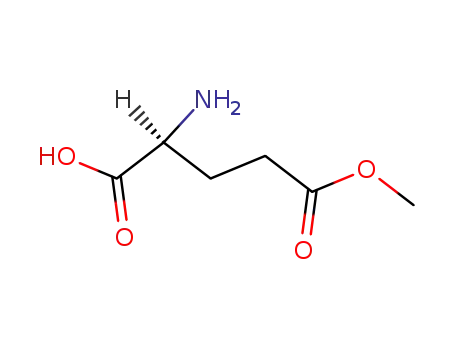
L-glutamic acid 5-methyl ester
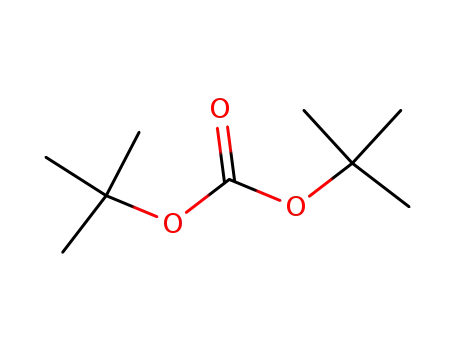
tert-butyldicarbonate
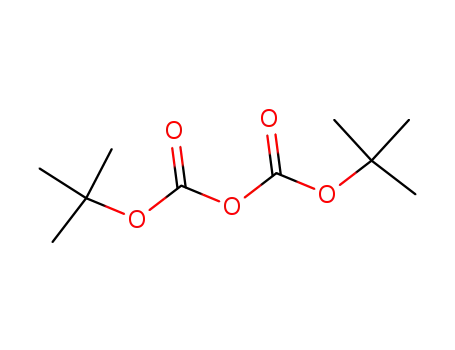
di-tert-butyl dicarbonate
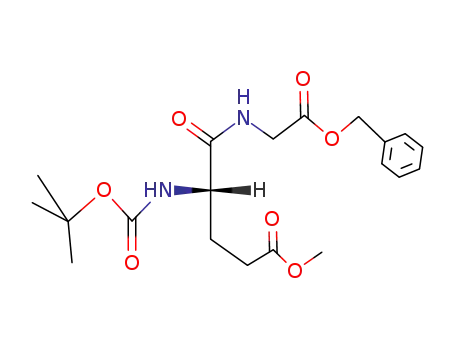
tert.-Butoxycarbonyl-(γ-O-methyl-Glu)-Gly-benzylester
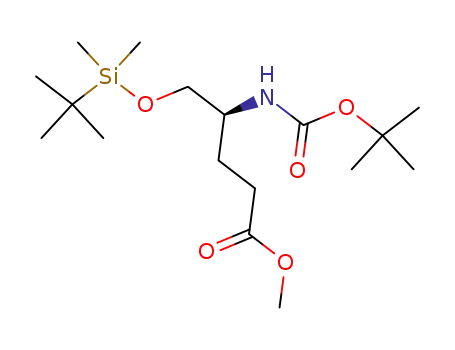
Methyl (4S)-4-(N-(tert-Butoxycarbonyl)amino)-5-((tert-butyldimethylsilyl)oxy)pentanoate
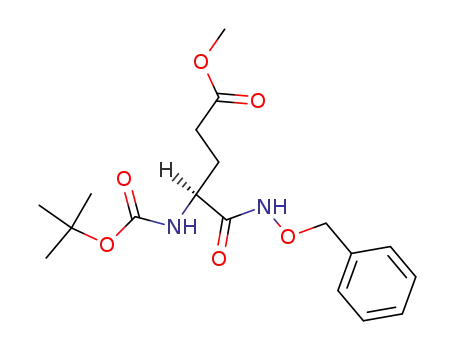
N-(Benzyloxy)-Nα-(tert-butoxycarbonyl)-L-glutamamide γ-methyl ester
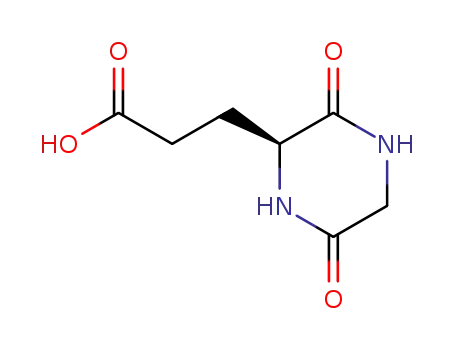
acide 3-(3,6-dioxopiperazin-2-yl)-propanoique
CAS:56-12-2
CAS:7531-52-4
CAS:102089-74-7
CAS:59279-60-6