Your Location:Home >Products >Biochemical Engineering >102089-74-7
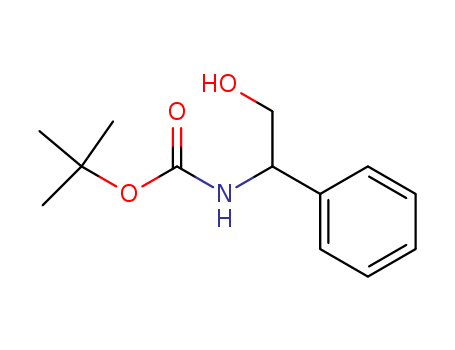

Product Details
|
Chemical Properties |
white to light yellow crystal powde |
|
Uses |
Chiral building block |
| European Community (EC) Number | 600-273-1 |
| DSSTox Substance ID | DTXSID00426847 |
Isomeric SMILES: CC(C)(C)OC(=O)N[C@@H](CO)C1=CC=CC=C1
InChIKey: IBDIOGYTZBKRGI-NSHDSACASA-N
InChI: InChI=1S/C13H19NO3/c1-13(2,3)17-12(16)14-11(9-15)10-7-5-4-6-8-10/h4-8,11,15H,9H2,1-3H3,(H,14,16)/t11-/m0/s1
An efficient and practical protocol for ...
Treatment of N-tert-butoxycarbonyl deriv...
Highly chemoselective amide forming liga...
p-Methoxybenzyl ethers were selectively ...
A convenient and useful protocol has bee...
An efficient ZnO nano catalyst, which wa...
With the increase of fungal infection an...
Metal-free, catalytic enantioselective i...
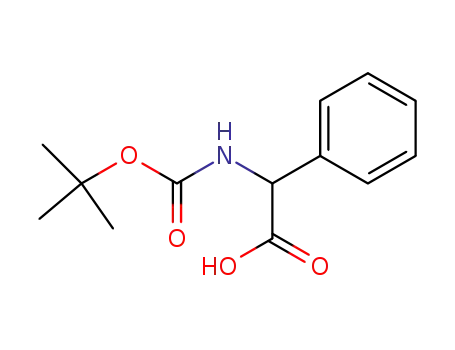
2-(tert-butoxycarbonylamino)-2-phenylacetic acid

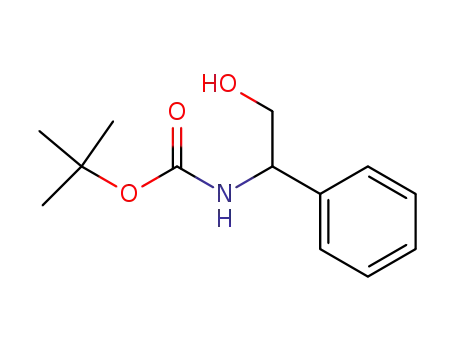
tert-butyl 2-hydroxy-1-phenylethylcarbamate
| Conditions | Yield |
|---|---|
|
2-(tert-butoxycarbonylamino)-2-phenylacetic acid; With borane-THF; In tetrahydrofuran; at 0 ℃;
With water; ammonium chloride; In tetrahydrofuran;
|
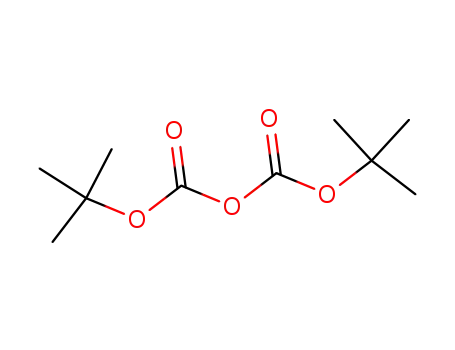
di-tert-butyl dicarbonate

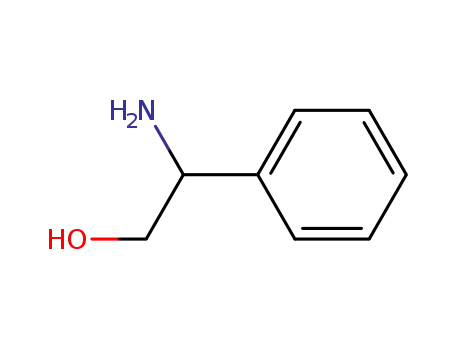
Phenylglycinol


tert-butyl 2-hydroxy-1-phenylethylcarbamate
| Conditions | Yield |
|---|---|
|
With triethylamine; In dichloromethane; at 20 ℃; for 6h;
|
91.7% |
|
With triethylamine; In dichloromethane; at 20 ℃;
|
90% |
|
With aminosulfonic acid; at 30 ℃;
|
84% |
|
With sodium hydroxide; In dichloromethane; Ambient temperature;
|
83% |
|
With sodium hydroxide; In diethyl ether; water;
|
|
|
In dichloromethane; at 20 ℃; for 3h;
|
|
|
With triethylamine; In dichloromethane; for 12h;
|
|
|
With potassium hydroxide;
|
|
|
With triethylamine; In dichloromethane; Cooling with ice;
|
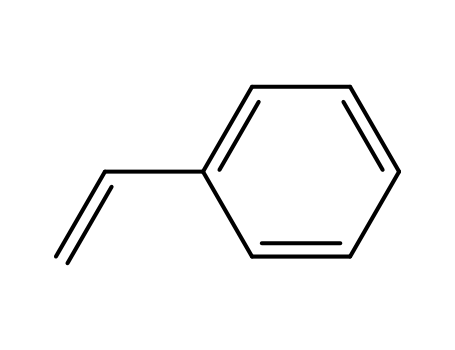
styrene
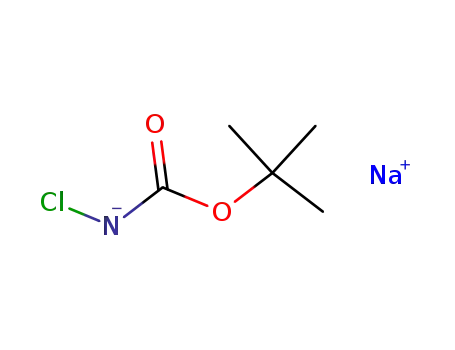
N-chloro-N-sodio-tert-butylcarbamate

di-tert-butyl dicarbonate
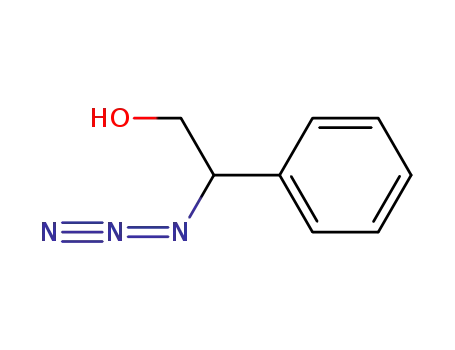
2-azido-2-phenylethanol
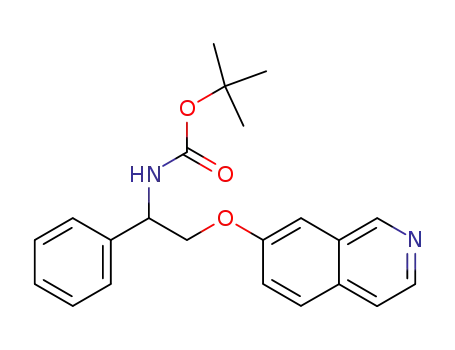
[2-(isoquinolin-7-yloxy)-1-phenyl-ethyl]-carbamic acid tert-butyl ester
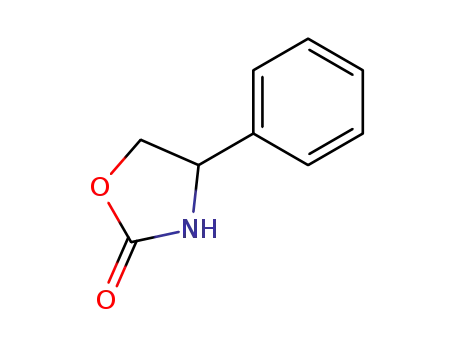
4-phenyl-2-oxazolidinone
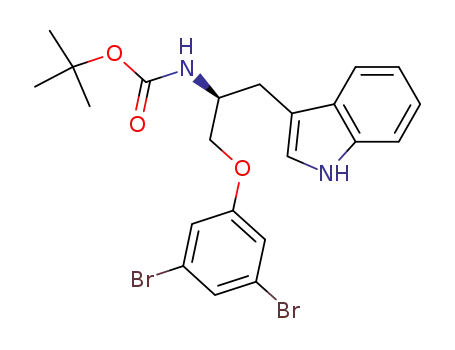
(2S)-[1-(3,5-dibromo-phenoxymethyl)-2-(1H-indol-3-yl)-ethyl]-carbamic acid tert-butyl ester
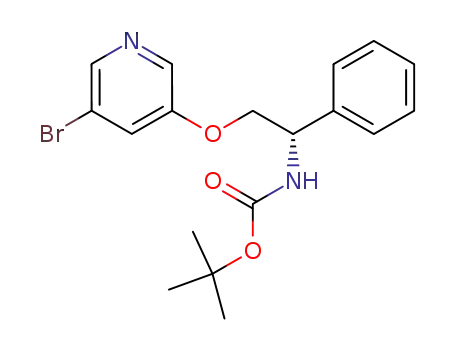
(2-hydroxy-1-phenyl-ethyl)-carbamic acid tert-butyl ester
CAS:56-12-2
CAS:7531-52-4
CAS:7274-88-6
CAS:45214-91-3