Your Location:Home >Products >Biochemical Engineering >7274-88-6
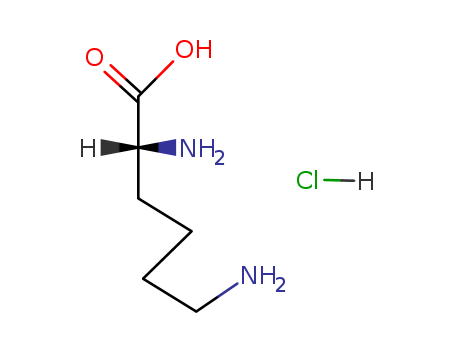

Product Details
|
Description |
D-lysine hydrochloride is the hydrochloride salt of D-lysine. It contains a D-lysine. D-lysine is a kind of D-form amino acid (not the natural amino acid in organisms, which is in L-form). |
|
Chemical Properties |
white crystalline powder |
|
Uses |
D-Lysine is used as a component of a wide array of polymers (poly-D-lysine), surfactants and biofilms to confer a positive (cationic) charge. |
|
Definition |
ChEBI: The hydrochloride salt of D-lysine. |
Isomeric SMILES: C(CCN)C[C@H](C(=O)O)N.Cl
InChIKey: BVHLGVCQOALMSV-NUBCRITNSA-N
InChI: InChI=1S/C6H14N2O2.ClH/c7-4-2-1-3-5(8)6(9)10;/h5H,1-4,7-8H2,(H,9,10);1H/t5-;/m1./s1
The synthesis and application of three c...
Therefore, in this work, hexathiobenzene (M-1) with photoactive aggregation-induced emission (AIE) properties were used as fluorescent dyes, and chiral amino acids L/D-Lysine hydrochloride (L/D-Lys) were used as chiral templates. Supramolecular L/D-Lys@M-1 components are formed by intermolecular hydrogen bonding in a mixed solvent (N,N-dimethylformamide (DMF)/H2O).
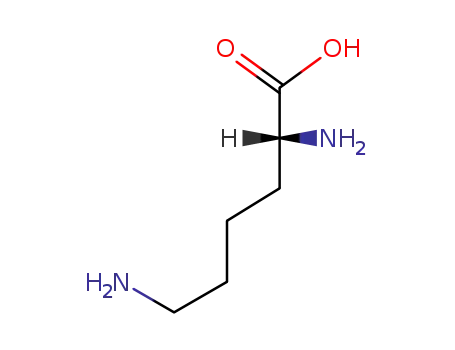
D-lysine

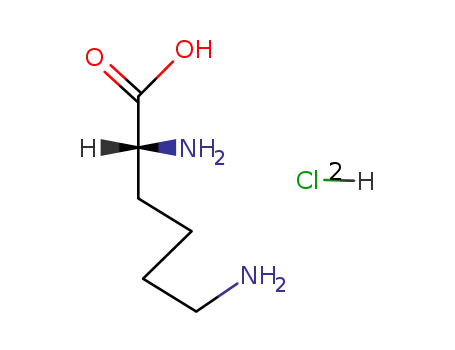
D-lysine bis(hydrochloride)
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In tetrahydrofuran; water; for 3h;
|
94.6% |
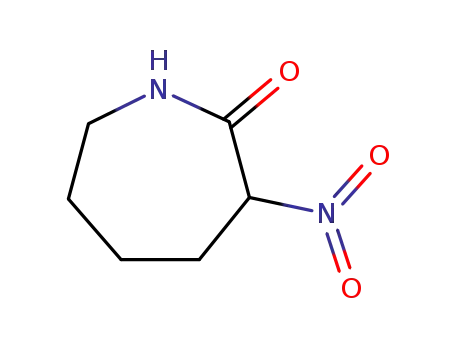
α-nitrocaprolactam

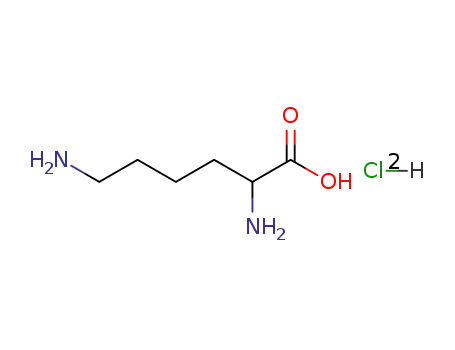
2,6-Diamino-hexanoic acid; hydrochloride

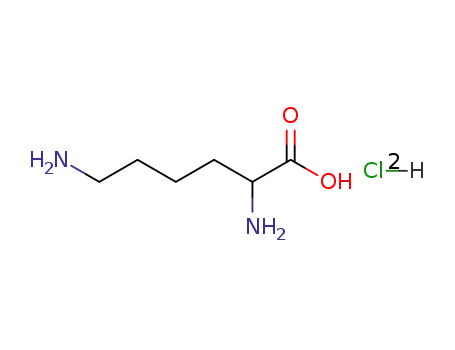
2,6-Diamino-hexanoic acid; hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; (S)-1-phenyl-ethylamine; hydrogen; palladium dichloride; Product distribution; 1.) DME, 20 deg C, 5 h;
|

D-lysine

α-nitrocaprolactam

2,6-Diamino-hexanoic acid; hydrochloride
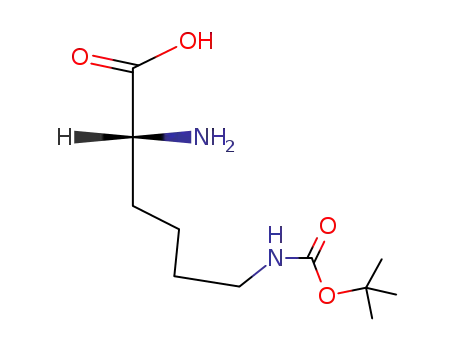
Nε-(tert-butoxycarbonyl)-D-lysine
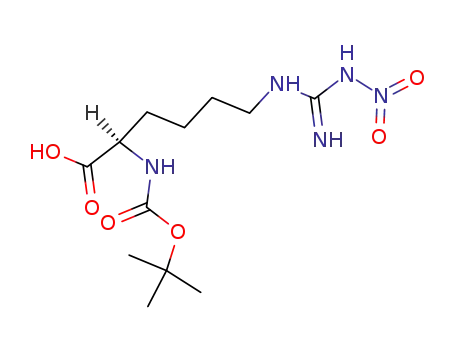
Boc-D-Har(NO2)-OH
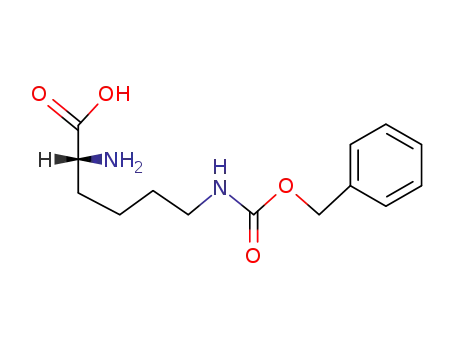
(R)-2-amino-6-(((benzyloxy)carbonyl)amino)hexanoic acid
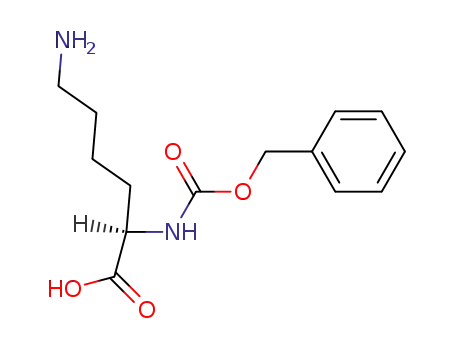
Nα-carbobenzoxy-D-lysine
CAS:138-15-8
CAS:86028-91-3
CAS:153-94-6
CAS:102089-74-7