Your Location:Home >Products >Biochemical Engineering >344-25-2
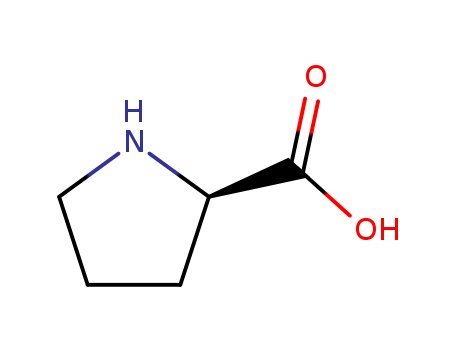

Product Details
|
Chemical Properties |
White to off-white powder |
|
Uses |
D-proline is an important chiral intermediate in the production of several serotonin analogs, such as eletriptan, which is an anti-migraine drug. Used in the synthesis of (R)-(+)-N-Boc-Pipecolic Acid, (S)-(-)-Coniine, (S)-(+)-Pelletierine, (+)-.beta.-Conhydrine, and (S)-(-)-Ropivacaine and formal synthesis of (-)-Lasubine II and (+)-Cermizine C. D-proline could trigger the dysfunction of bacterial cells and affect bacterial reproduction. d-proline can be used as the reducing and capping agent to prepare fluorescent gold nanoclusters. |
|
Definition |
ChEBI: The D-enantiomer of proline. |
Isomeric SMILES: C1C[C@@H](NC1)C(=O)O
InChIKey: ONIBWKKTOPOVIA-SCSAIBSYSA-N
InChI: InChI=1S/C5H9NO2/c7-5(8)4-2-1-3-6-4/h4,6H,1-3H2,(H,7,8)/t4-/m1/s1
In addition, the desired products with opposite configurations can be easily obtained by employing cheap and commercially available L-/D-proline as chiral organocatalysts.
The synthesis of d-proline-derived hydroxamic acids containing diverse appendages at the amino group, varying in length and decoration allowed to give insight on the MMP2/MMP9 …
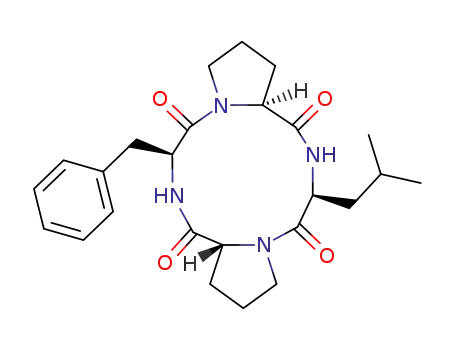
cyclo-(L-Pro-L-Leu-D-Pro-L-Phe)

L-leucine

L-phenylalanine

L-proline

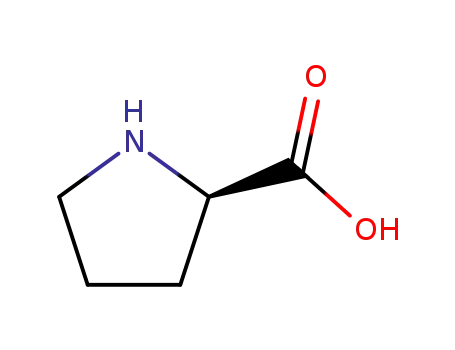
D-Prolin
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 110 ℃; for 24h;
|
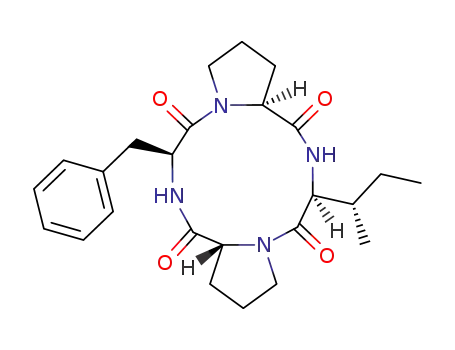
cyclo-(Pro-Ile-Pro-Phe)

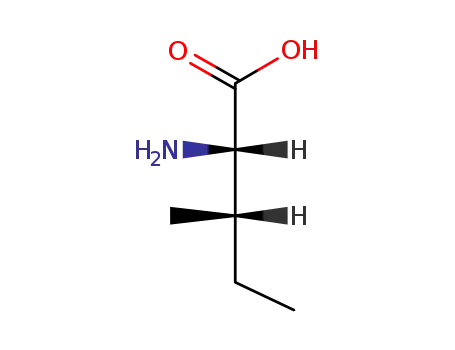
L-isoleucine


L-phenylalanine

L-proline


D-Prolin
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 110 ℃; for 24h;
|
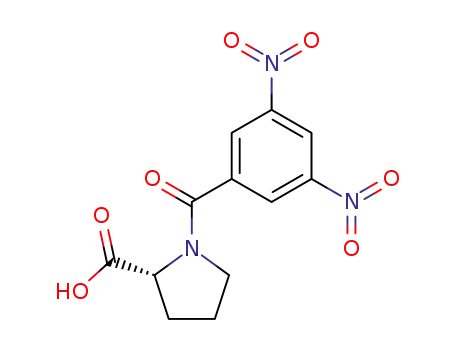
(R)-N-(3,5-dinitrobenzoyl)proline
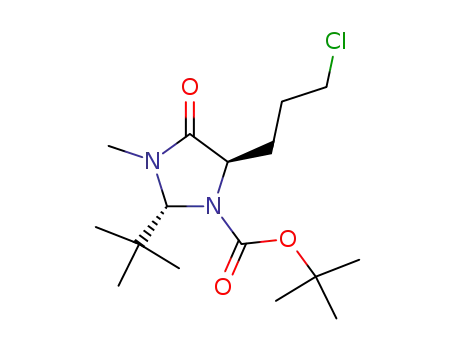
(2R,5R)-2-t-butyl-1-t-butyloxycarbonyl-5-(3-chloropropyl)-3-methyl-4-imidazolidinone
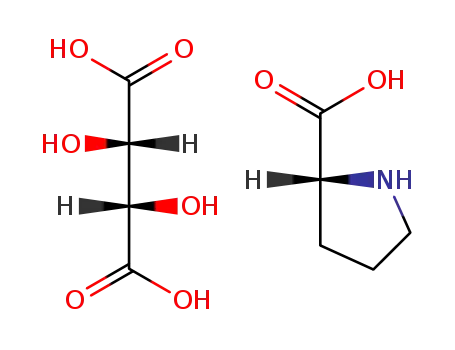
(2S,3S)-2,3-Dihydroxy-succinic acid; compound with (R)-pyrrolidine-2-carboxylic acid
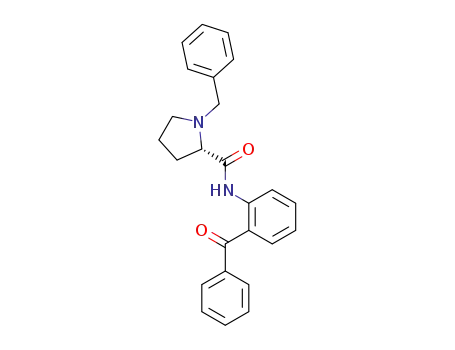
(S)-N-(2-benzoylphenyl)-1-benzylpyrrolidine-2-carboxamide
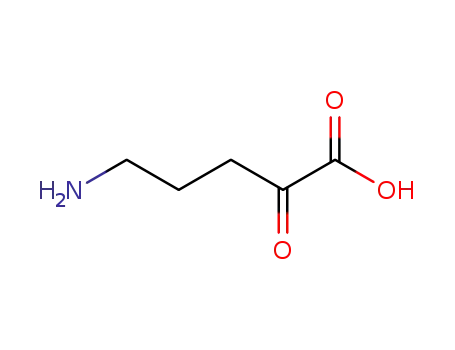
5-amino-2-oxo-valeric acid
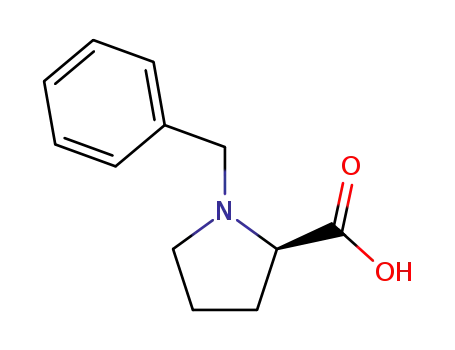
(D)-1-benzylpyrrolidine-2-carboxylic acid
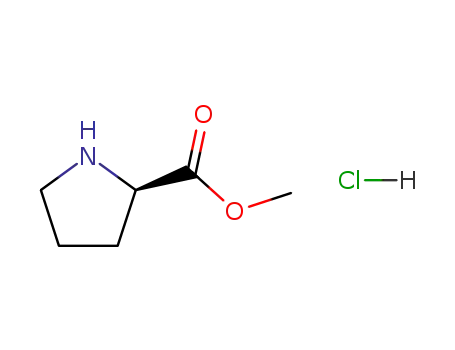
D-proline methyl ester hydrochloride
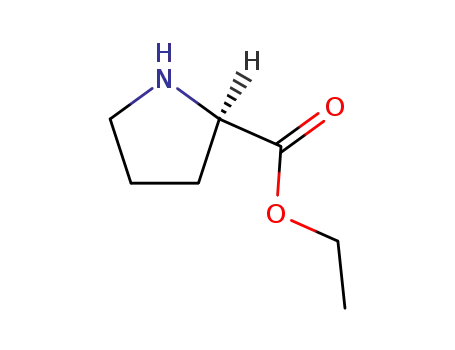
ethyl proline hydrochloride
CAS:56-12-2
CAS:7531-52-4
CAS:640-68-6
CAS:5959-95-5