Your Location:Home >Products >Biochemical Engineering >640-68-6

Product Details
|
Description |
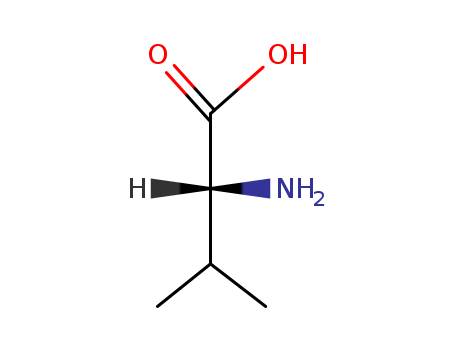
D-valine is the D-form of valine, being the non-proteinogenic isomer of valine. It can be supplemented into the cell culture for selectively inhibition of cell proliferation through inhibiting cells that are deficient in the enzyme D-amino acid oxidase. |
|
Uses |
D-valine can be used for the synthesis of newly efficient pesticide: pyrethroids permethrin and chlorofluorocarbons amyl because of its own biochemical characteristics. D-Valine is an important organic chiral source which is mainly used in fields such as chiral pharmaceuticals, chiral additive, and chiral auxiliary and other areas. D-valine is the intermediates for fluvalinate. It is used as a pharmaceutical raw materials and pharmaceutical intermediates, and can also be used for synthetic sweeteners Alatan. It can also be used for biochemical research. D-valine hydrogel can reduce inflammatory factor level, reduce alveolar bone resorption, and improve periodontitis, which may be related to periodontal pathogens. |
|
Chemical Properties |
White crystal, m.p.> 295 °C (sublimation), [α] 25 = 27.35°; it is soluble in water and very slightly soluble in ethanol. |
|
Production methods |
1. DL-Acetyl-methionine is used as the raw material. It undergoes acylase splitting, and further hydrochloric acid acidification to have D-valine crystals precipitated; refined product is finally obtained through recrystallization. 2. The preparation method is to use 2-isopropyl-acetyl ethyl to react with benzene diazonium to get corresponding hydrazine compound, and then further reduce it to valine in zinc-ethanol solution and finally go through chemical or biological split. |
|
Definition |
ChEBI: The D-enantiomer of valine. |
|
General Description |
L-Valine is an essential non-polar amino acid. D-Valine is the non-proteinogenic isomer of valine. The demand for D-Valine increases because of its wide range of use. |
Isomeric SMILES: CC(C)[C@H](C(=O)O)N
InChIKey: KZSNJWFQEVHDMF-SCSAIBSYSA-N
InChI: InChI=1S/C5H11NO2/c1-3(2)4(6)5(7)8/h3-4H,6H2,1-2H3,(H,7,8)/t4-/m1/s1
This method provides D-Valine with optical purity of 97% and an overall yield of 72%. Furthermore, the immobilized cells can be reused for more than 7 cycles and maintain their capacity of over 70%. Hence the immobilized cells of engineered strain HC01 could potentially be used to prepare D-Valine.
Enantiomerically pure d-amino acids are ...
In this work, efficient NLO single crystal of L-Isoleucine D-Valine (LIDV) was grown successfully by employing slow evaporation method-the simplest method. …
itralamide B

N-methylalanine

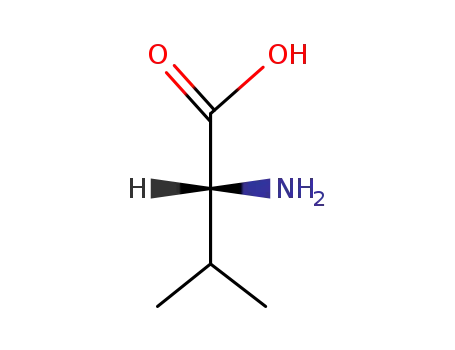
D-Val-OH

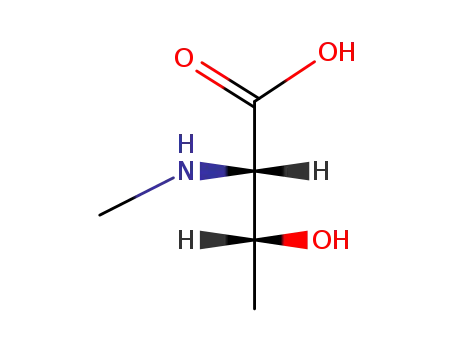
(2S,3R)-3-hydroxy-2-(methylamino)butanoic acid

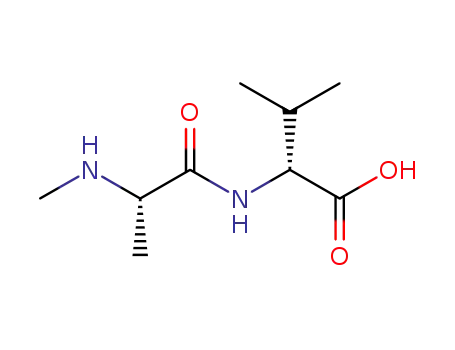
N-methylalanylvaline

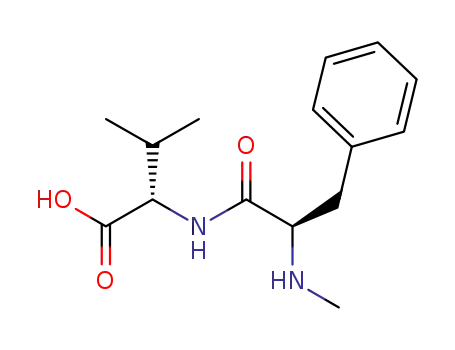
N-methylphenylalanylvaline

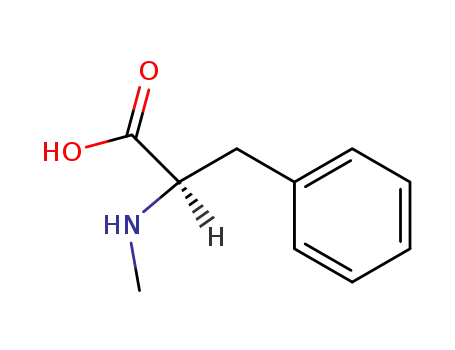
N-methyl-D-phenylalanine
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; water; at 110 ℃; for 18.5h;
|
![(4aS,10aR,13S,19aR,21aS,26bR,27aS,30R)-24-chloro-2,3,4,4a,8,9,10,10a,12,13,17,18,19,19a,22,26b,27,27a,29,30-icosahydro-25,26b-dihydroxy-12,13-dimethyl-30-(propan-2-yl)-7H,21aH-tripyridazino[1’’,6’’:10’,11’;1’’’,6’’’:16’,17’;1’’’’,6’’’’:7’,8’] [1,4,7,10,13,16]hexaazacyclooctadecino[1’,2’:1,5]pyrrolo[2,3-b]indole-5,11,14,20,28,31(1H,16H)-hexone](/upload/2023/6/8f2b76eb-b580-4a64-b266-50f07a732c8d.png)
(4aS,10aR,13S,19aR,21aS,26bR,27aS,30R)-24-chloro-2,3,4,4a,8,9,10,10a,12,13,17,18,19,19a,22,26b,27,27a,29,30-icosahydro-25,26b-dihydroxy-12,13-dimethyl-30-(propan-2-yl)-7H,21aH-tripyridazino[1’’,6’’:10’,11’;1’’’,6’’’:16’,17’;1’’’’,6’’’’:7’,8’] [1,4,7,10,13,16]hexaazacyclooctadecino[1’,2’:1,5]pyrrolo[2,3-b]indole-5,11,14,20,28,31(1H,16H)-hexone

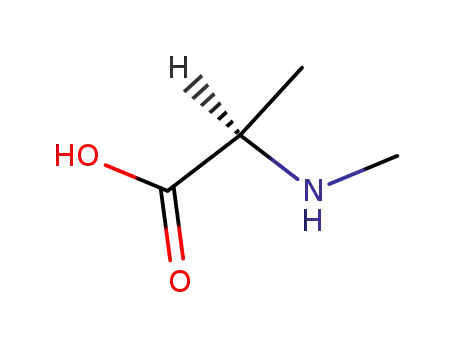
N-methyl-D-alanine


D-Val-OH

![(2S,3aR,8aR)-6-chloro-1,2,3,3a,8,8a-hexahydro-3a,5-dihydroxypyrrolo-[2,3-b]indole-2-carboxylic acid](/upload/2023/6/b28d2601-af26-4e00-bf34-3cdbb51e9ef0.png)
(2S,3aR,8aR)-6-chloro-1,2,3,3a,8,8a-hexahydro-3a,5-dihydroxypyrrolo-[2,3-b]indole-2-carboxylic acid

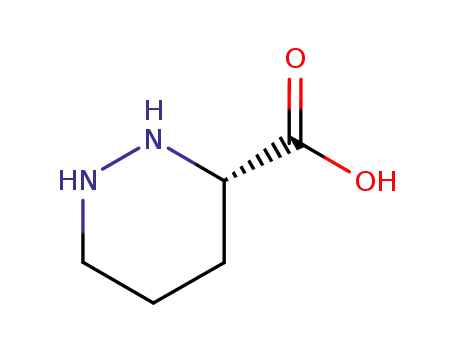
(3S)-hexahydropyridazine-3-carboxylic acid
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; water; at 110 ℃; for 24h;
|
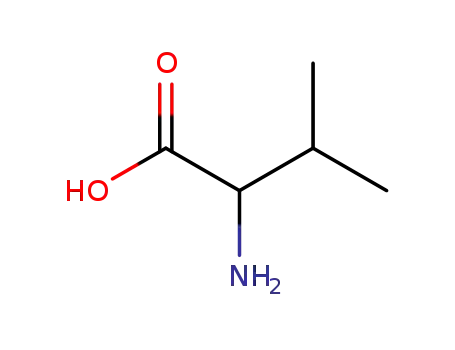
D,L-valine
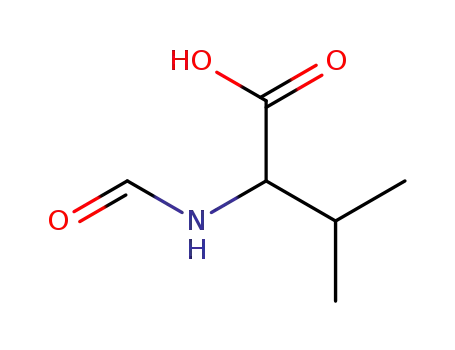
N-formylvaline
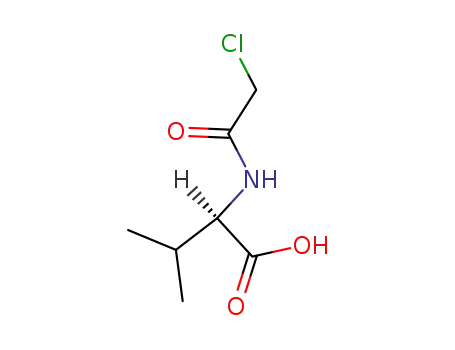
N-chloroacetyl-D-valine
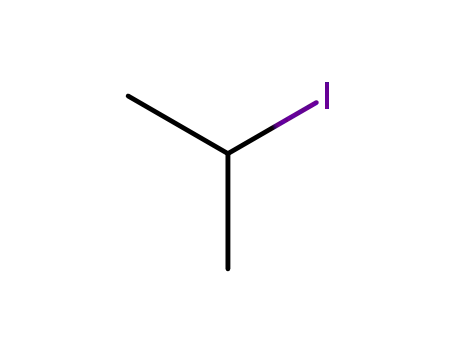
2-iodo-propane
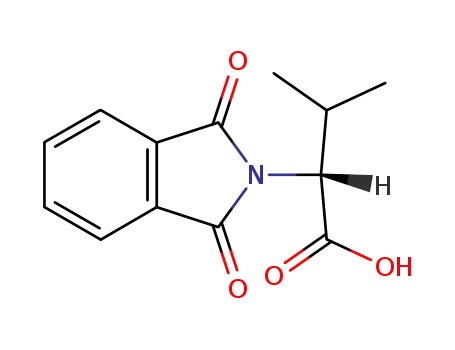
(R)-N-phthaloylvaline
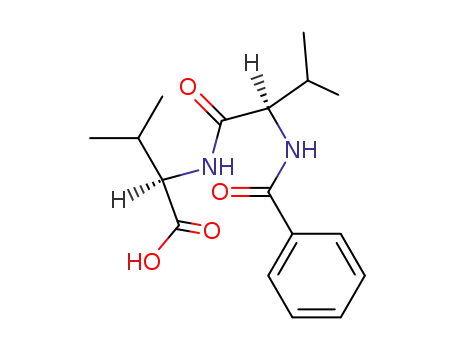
N-(N-benzoyl-D-valyl)-D-valine
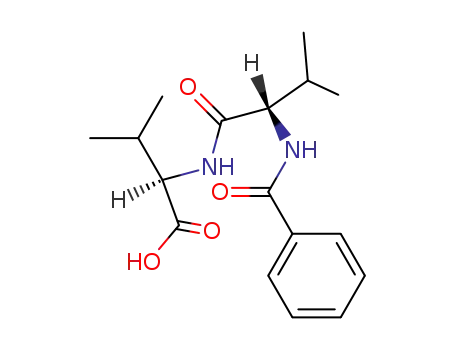
N-(N-benzoyl-L-valyl)-D-valine
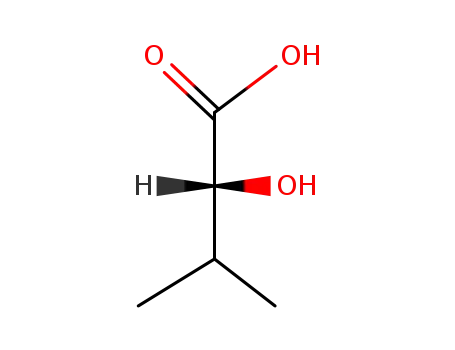
(R)-2-Hydroxy-3-methylbutyric acid
CAS:56-12-2
CAS:7531-52-4
CAS:351-50-8
CAS:344-25-2