Your Location:Home >Products >Biochemical Engineering >351-50-8
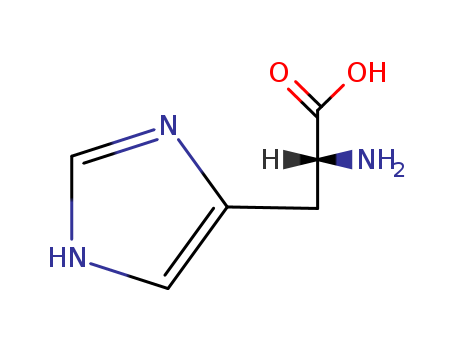

Product Details
|
Chemical Properties |
white crystalline powder |
|
Uses |
D-Histidine is the unnatural, biologically inactive isomer of L-Histidine (H456010). D-histidine is known to inhibit cell division, and is also used by certain types of bacteria (such as Escherichia coli) as a source of L-Histidine. |
|
Definition |
ChEBI: An optically active form of histidine having D-configuration. D-histidine has a typical absorption peak at about 2.8 THz. |
|
Biochem/physiol Actions |
D-Histidine may be used in the design of peptide drugs, cationic peptides, such as analogues of carnosine. D-Histidine may also be used as a heavy metal sequestration agent. D-Histidine effectively inhibits Streptococcus mutan biofilm formation in a dose-dependent manner and D-Histidine interferes with the viability of Streptococcus mutans. |
InChI:InChI=1/C6H9N3O2/c7-5(6(10)11)1-4-2-8-3-9-4/h2-3,5H,1,7H2,(H,8,9)(H,10,11)/t5-/m1/s1
In this study, D-arginine (R), D-methionine (M), D-histidine (H), and a mixture of these D-amino acids (D-AAs) were investigated as an effective therapeutic strategy against P. gingivalis biofilms.
Three new chiral stationary phases (CSPs...
Complex formation in the copper(II) – glycylglycyl-l-tyrosine and copper(II) – glycylglycyl-l-tyrosine – l/d-histidine systems was investigated by pH-potentiometric titration and spectrophotometry methods in the pH range 2–11.
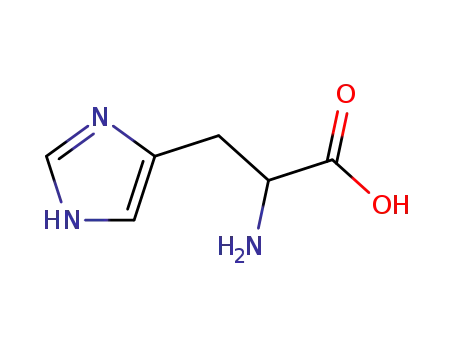
D,L-histidine

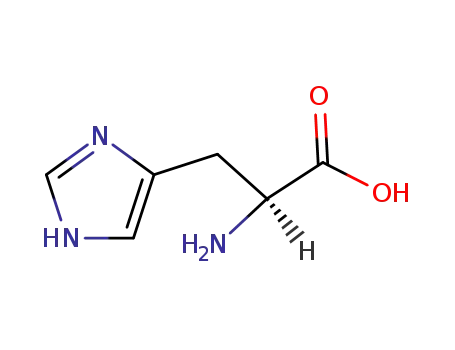
L-histidine

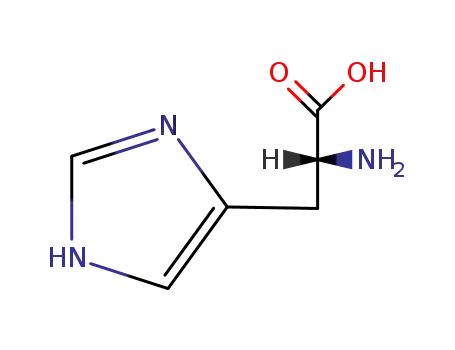
D-histidin
| Conditions | Yield |
|---|---|
|
|
|
|
With borane-ammonia complex; oxygen; ammonium formate; L-amino acid oxidase; In water; for 5h; pH=6.7;
|
|
|
With 1-hexyl-3-methylimidazolium L-prolinate; copper(II) acetate monohydrate; In methanol; water; at 25 ℃; pH=5.8; Resolution of racemate; solid phase reaction;
|
|
|
With sulfuric acid; In methanol; water; at 25 ℃; Resolution of racemate;
|
|
|
With conjugated polyfluorene appended with protected L-glutamic acid; In water; for 48h; enantioselective reaction; Resolution of racemate;
|
49.55 % ee |
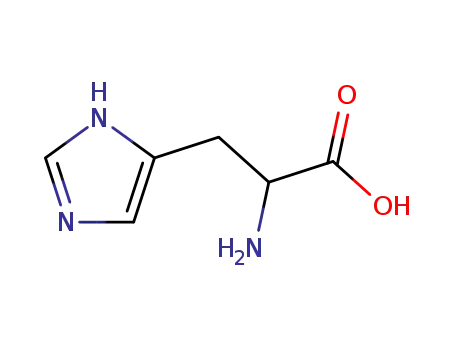
His


L-histidine


D-histidin
| Conditions | Yield |
|---|---|
|
durch fraktionierte Krystallisation des d-weinsaeuren Salzes; das Salz des d-Histidins krystallisiert zuerst aus;
|

His

L-histidine

D,L-histidine
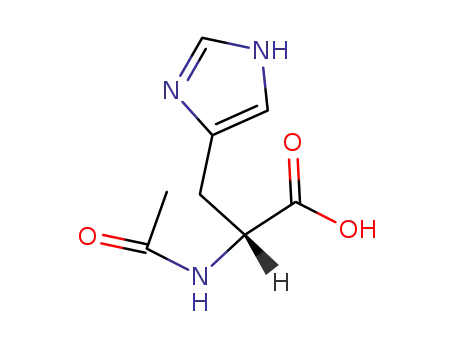
Nα-acetyl-D-histidine
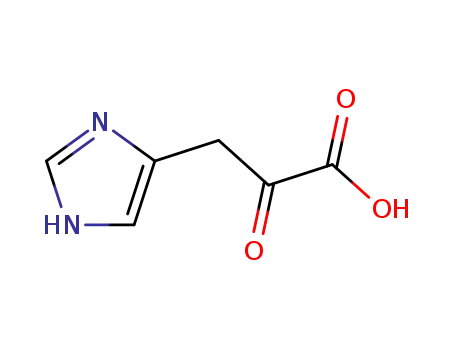
β-imidazolyl pyruvic acid
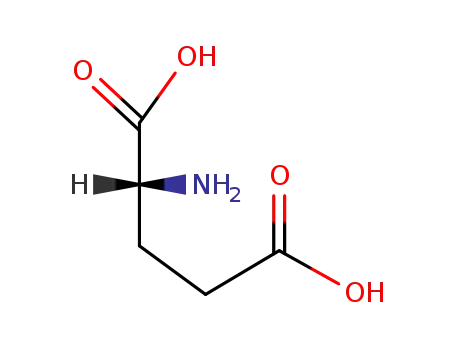
D-Glutamic acid

L-histidine
(R)-histidine methyl ester dihydrochloride
CAS:56-12-2
CAS:7531-52-4
CAS:312-84-5
CAS:640-68-6