Your Location:Home >Products >Biochemical Engineering >312-84-5

Product Details
|
Overview |
D-serine is the D-form of the amino acid serine, but is not used for the protein synthesis. |
|
Localization |
D-Serine has been detected at relatively high levels in certain areas in the adult brain with particularly high levels of nMdars, including cerebral cortex, hippocampus, thalamus, hypothalamus, amygdala, and retina. |
|
Synthesis and Metabolism |
Humans can acquire D-serine through ingestion with food, derivation from gastrointestinal bacteria, liberation from metabolically stable proteins, which contain D-amino acids after racemization with ageing, and through biosynthesis from L-serine. |
|
Relation with diseases |
As it is involved in nMdar neurotransmission in the brain, nMdar-dependent plasticity, and developmental processes, it is not astonishing that dysregulation of d-serine signaling might also be involved in several pathologies, including neuropsychiatric and neurodegenerative diseases related to nMdar dysfunction. Intense stimulation of nMdars has been associated with considerable number of acute and chronic neurodegenerative conditions, including stroke, epilepsy, polyneuropathies, chronic pain, amyotrophic lateral sclerosis(ALS), Parkinson’s disease(PD), Alzheimer’s disease(AD), and Huntington’s disease(HD)[42]. |
|
Description |
Natural D-serine (D-Ser) has been detected in animals more than two decades ago, but little is known about the physiological functions of D-Ser. |
|
Chemical Properties |
D-serine is a off-white crystalline powder with a faint musty odor. |
|
Uses |
Clinical studies have been conducted in schizophrenia patients to evaluate body fluid levels of D-serine and/or to use D-serine alone or in combination with antipsychotics to determine its effectiveness as a therapeutic agent. D-serine has also been used in combination with DAAO inhibitors in preclinical investigations, and interesting results have been obtained. D-serine is now garnering attention as the primary NMDAR co-agonist in limbic brain regions implicated in neuropsychiatric disorders. L-serine is synthesized by astrocytes, which is then transported to neurons for conversion to D-serine by serine racemase (SR), a model we term the ‘serine shuttle.’ |
|
Definition |
D-serine is the R-enantiomer of serine. It has a role as a NMDA receptor agonist, a human metabolite and an Escherichia coli metabolite. It is a D-alpha-amino acid and a serine. It is a conjugate base of a D-serinium. It is a conjugate acid of a D-serinate. It is an enantiomer of a L-serine. It is a tautomer of a D-serine zwitterion. |
|
Application |
D-serine has been used as a substrate in D-amino acid oxidase (DAO) activity in human 1321N1 astrocytoma cells. It has also been used in intracerebroventricular administration in rat for the induction of antinociceptive effect.D-serine has been used to prevent glycine-dependent desensitization of N-methyl D-aspartate receptor (NMDAR) and to study its effects on NMDARs to correct behavioral abnormalities in rats after partial sciatic nerve ligation (PSNL). |
|
General Description |
D-serine is an unusual amino acid expressed in the mammalian brain. |
|
Biological Activity |
d-Serine, a free amino acid synthesized by serine racemase, is a coagonist of N-methyl-d-aspartate–type glutamate receptor (NMDAR). |
|
Biochem/physiol Actions |
D-serine is an agonist and glycine mimic which is active at the strychnine-insensitive glycine binding site associated with the N-methyl-D-aspartate (NMDA) receptor as well as the inhibitory post-synaptic glycine receptor. Along with glutamate, it has a role in various physiological processes including synaptic plasticity and receptor transmission. Dysregulation of D-serine signaling has been linked with neurodegenerative diseases and disorders. |
Isomeric SMILES: C([C@H](C(=O)O)N)O
InChIKey: MTCFGRXMJLQNBG-UWTATZPHSA-N
InChI: InChI=1S/C3H7NO3/c4-2(1-5)3(6)7/h2,5H,1,4H2,(H,6,7)/t2-/m1/s1
D-serine action requires binding to neuronal NMDA-type glutamate receptors. Fly astrocytes contribute processes to tripartite synapses, and the proportion of astrocytes that are themselves activated by glutamate increases with water deprivation.
In the case of skin injury, d-serine participates in homeostasis maintenance and recovery; a flux of cations mediated through NMDARs allows lamellar granule exocytosis from the keratinocytes, leading to skin permeabilization.
An efficient enzymatic method catalyzed ...
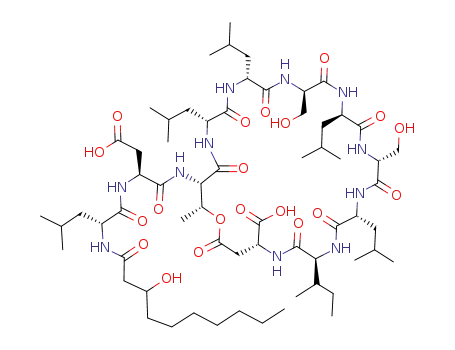
pholipeptin

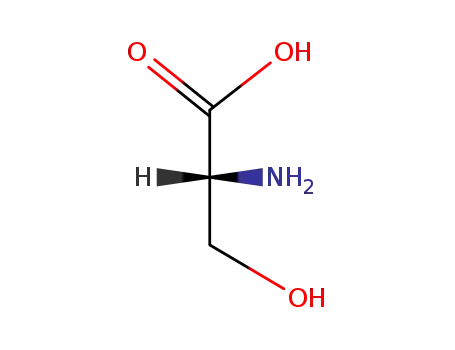
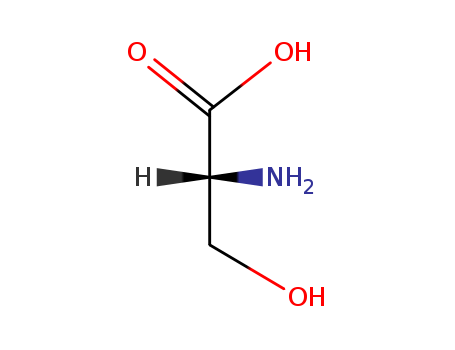
D-Serine

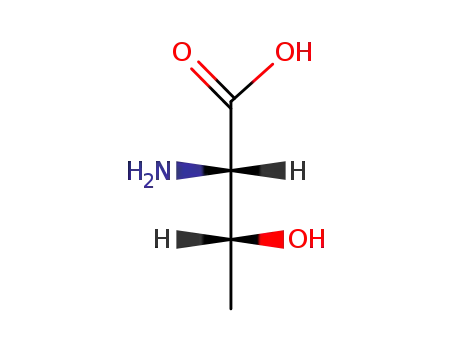
L-threonine

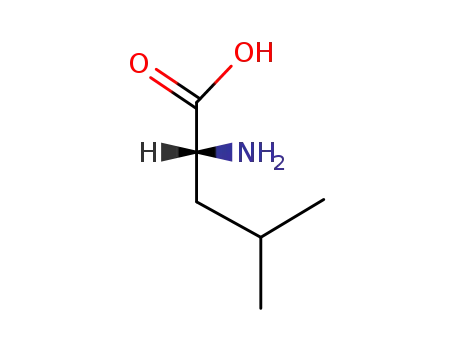
(R)-leucine

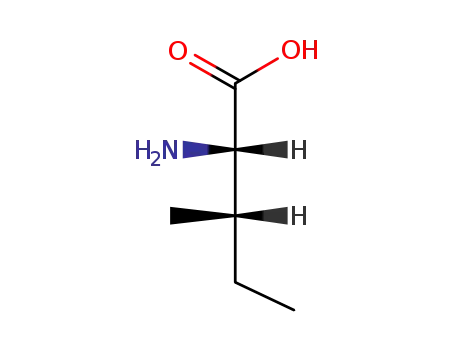
L-isoleucine

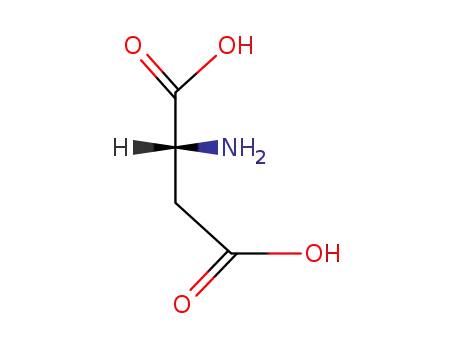
(2R)-aspartic acid

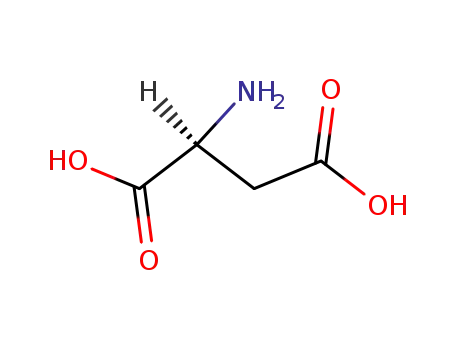
L-Aspartic acid
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; at 120 ℃; for 20h; Product distribution;
|
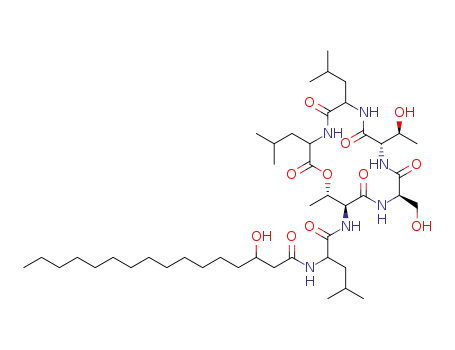
ngercheumicin I


D-Serine

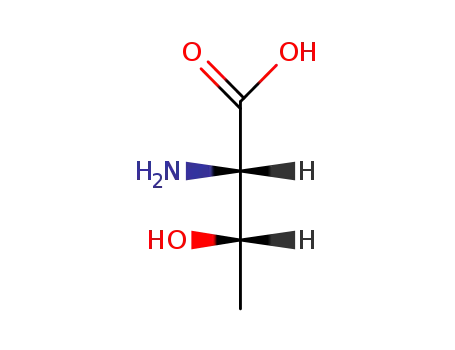
(2S,3S)-2-amino-3-hydroxybutanoic acid

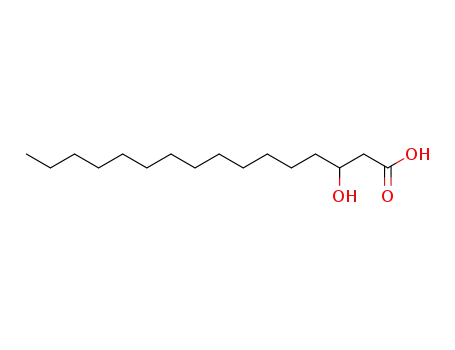
β-hydroxypalmitic acid

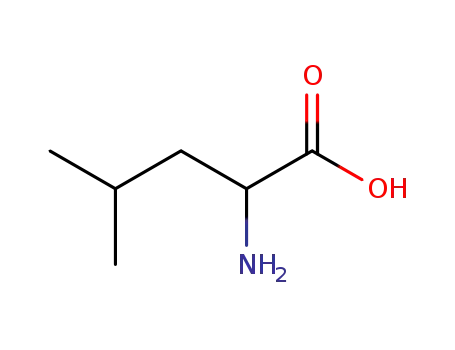
LEUCINE
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 110 ℃; for 20h;
|
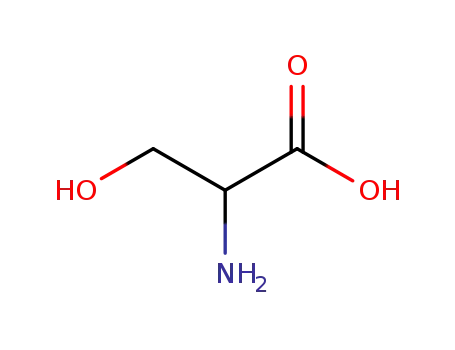
serin
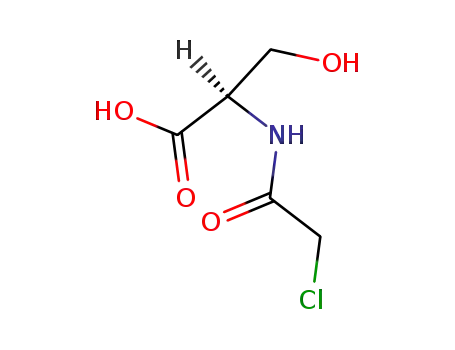
N-chloroacetyl-D-serine
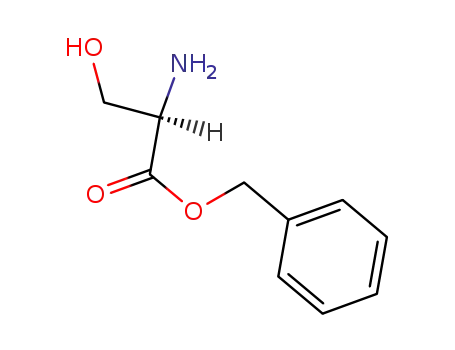
D-serine benzyl ester
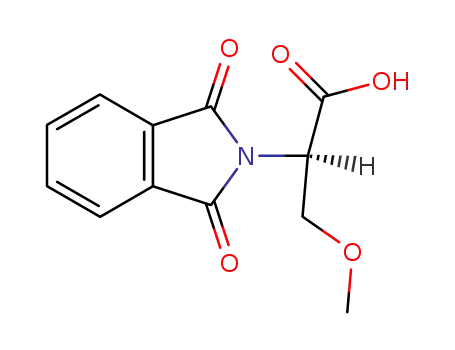
(R)-2-(isoindolin-2-yl)-3-methoxypropanoic acid
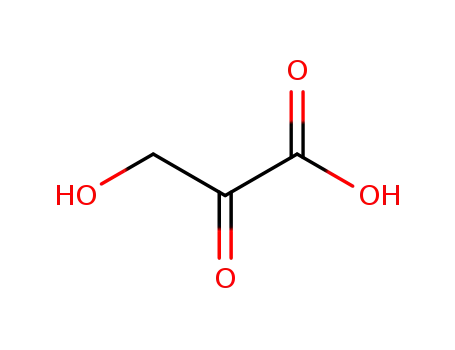
3-hydroxy-2-oxopropionic acid
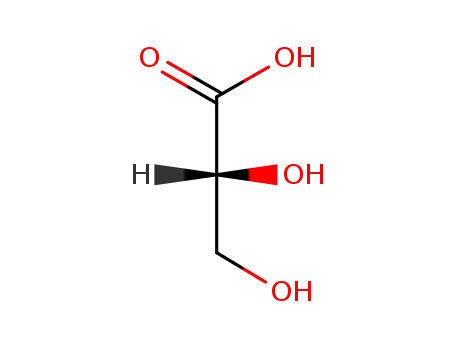
D-glyceric acid
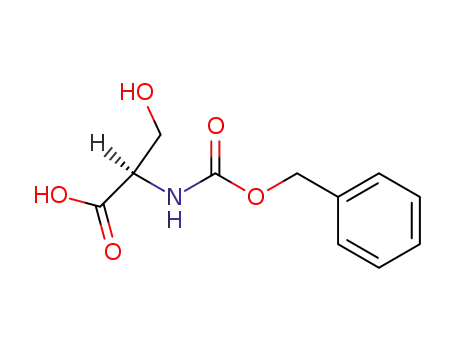
N-(benzyloxycarbonyl)-D-serine
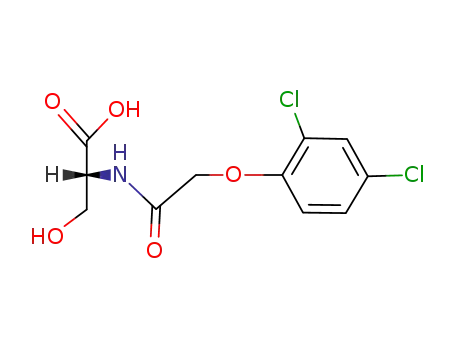
N-[(2,4-dichloro-phenoxy)-acetyl]-D-serine
CAS:56-12-2
CAS:7531-52-4
CAS:556-02-5
CAS:351-50-8