Your Location:Home >Products >Biochemical Engineering >19764-30-8

Product Details
|
Chemical Properties |
White to off-white powder |
| Uses | N-Acetyl-D-leucine is a metabolite found in or produced by Saccharomyces cerevisiae. It is used to help differentiate members of the amidohydrolase enzyme superfamily. |
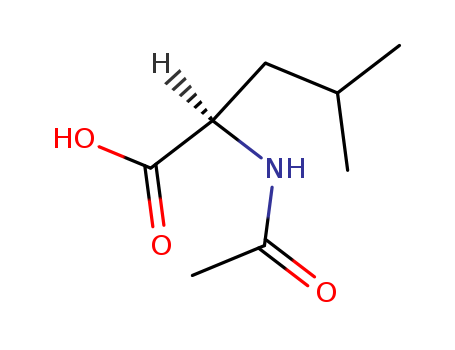
N-acetyl-D-leucine is the N-acetyl derivative of D-leucine. It is a N-acetyl-D-amino acid and a D-leucine derivative.
Isomeric SMILES: CC(C)C[C@H](C(=O)O)NC(=O)C
InChIKey: WXNXCEHXYPACJF-SSDOTTSWSA-N
InChI: InChI=1S/C8H15NO3/c1-5(2)4-7(8(11)12)9-6(3)10/h5,7H,4H2,1-3H3,(H,9,10)(H,11,12)/t7-/m1/s1
Although the N-acetyl-D-leucine is not reported to be toxic, concerns about the toxicity of D-amino acids in general have been raised as the reason for the original evolutionary selection and biological presence of D-amino acid oxidase.
Moreover, our recent findings that the enantiomers of N-acetyl-leucine (N-acetyl-L-leucine and N-acetyl-D-leucine) show unexpected and large differences in pharmacokinetics suggesting the involvement of differential binding sites provided by enzymes and transporters...
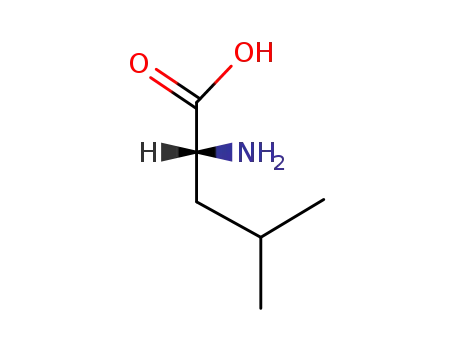
(R)-leucine

4-nitrophenol acetate

4-nitro-phenol

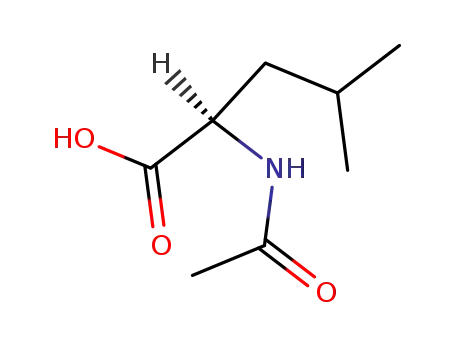
N-acetyl-D-leucine
| Conditions | Yield |
|---|---|
|
β‐cyclodextrin; In water; dimethyl sulfoxide; at 25 ℃; Rate constant; Mechanism; various conditions;
|
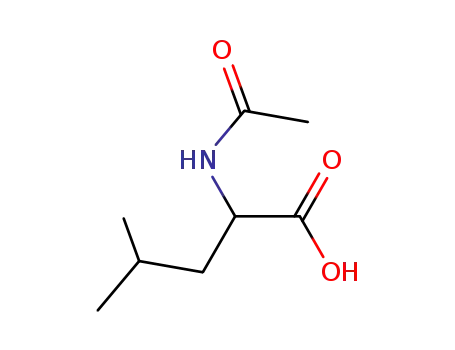
N-acetylleucine

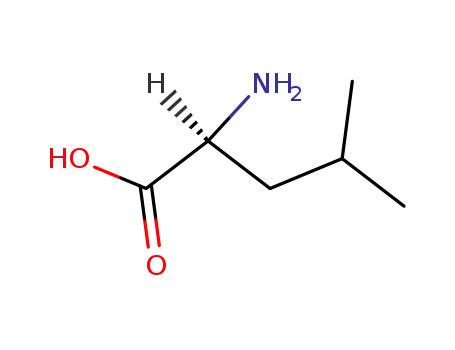
L-leucine


N-acetyl-D-leucine
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; potassium phosphate buffer; porcine kidney acylase I; at 40 ℃; relative initial rate of hydrolysis;
|
|
|
With polymer-supported Thermococcus litoralis L-aminoacylase; at 20 ℃; pH=8; stereoselective reaction; aq. buffer; Enzymatic reaction;
|
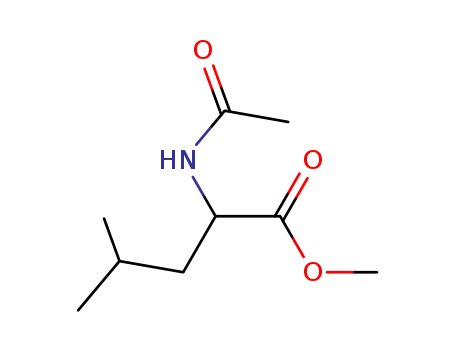
methyl N-acetyl-leucinate

N-acetylleucine
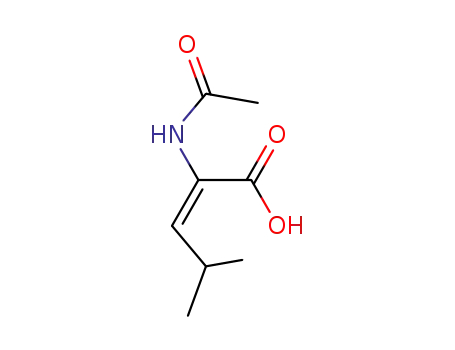
2-acetylamino-4-methyl-pent-2-enoic acid
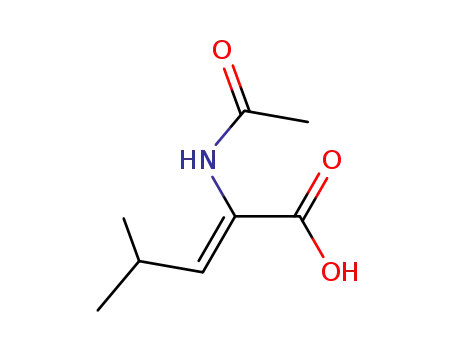
(Z)-2-Acetylamino-4-methyl-pent-2-enoic acid

(R)-leucine
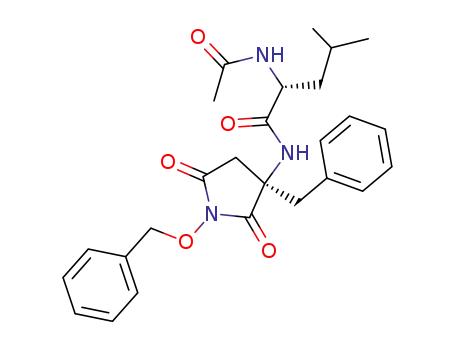
(R)-2-Acetylamino-4-methyl-pentanoic acid ((R)-3-benzyl-1-benzyloxy-2,5-dioxo-pyrrolidin-3-yl)-amide
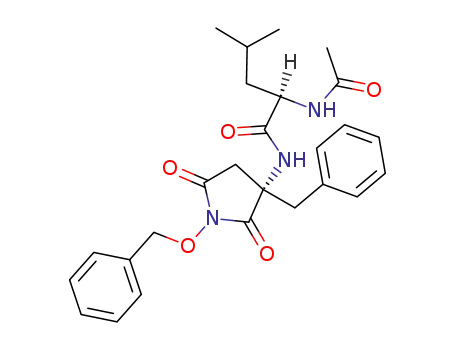
(R)-2-Acetylamino-4-methyl-pentanoic acid ((S)-3-benzyl-1-benzyloxy-2,5-dioxo-pyrrolidin-3-yl)-amide
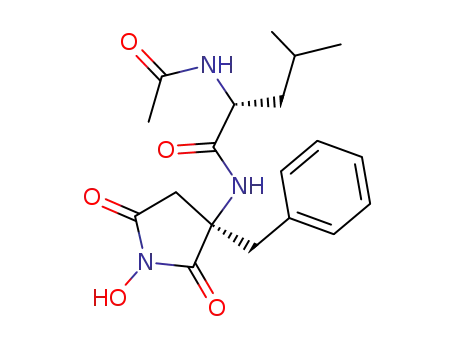
(+)-(3R)-3-(N-acetyl-D-leucylamino)-3-benzyl-1-hydroxysuccinimide
CAS:56-12-2
CAS:7531-52-4
CAS:1188-21-2
CAS:6893-26-1