Your Location:Home >Products >Biochemical Engineering >10009-20-8
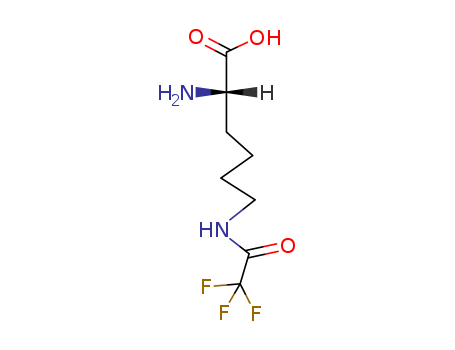

Product Details
|
Chemical Properties |
Off-White Solid |
|
Uses |
N(6)-trifluoroacetyl-L-lysine is an N(6)-acyl-L-lysine where the N(6)-acyl group is trifluoroacetyl. Used in the synthesis of new arborescent architectures of poly(L-lysine), called lysine dendrigraft (DGL) polymers. The amino acid N 6 -trifluoroacetyl-l-lysine is similar to TMSK but designed for the detection of the CF 3 group by 19 F NMR spectroscopy. |
|
Definition |
ChEBI: An N6-acyl-L-lysine where the N6-acyl group is trifluoroacetyl. |
InChI:InChI=1/C8H13F3N2O3/c9-8(10,11)7(16)13-4-2-1-3-5(12)6(14)15/h5H,1-4,12H2,(H,13,16)(H,14,15)/t5-/m0/s1
In this work, we improved the reported aqueous emulsion method for the synthesis of water-stable cesium lead bromide PNCs using CsBr, PbBr2, N-6-trifluoroacetyl-l-lysine, and oleylamine (OLA) as the raw materials.
Lysyl oxidase (LOX) is implicated in sev...
The polymer was obtained by reaction of MeOPegNH 2 with N6-trifluoroacetyl-l-lysine N-carboxyanhydride, followed by deprotection of the ω-amino groups and their functionalization …
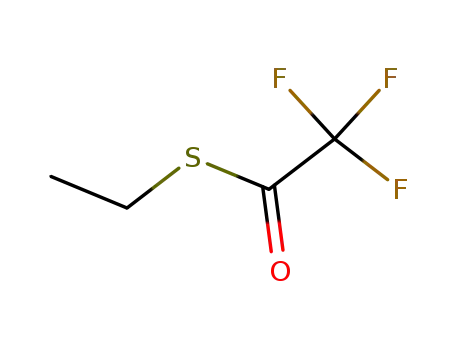
S-ethyl trifluoroacetate

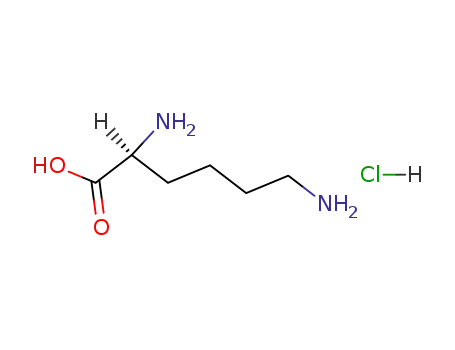
L-Lysine hydrochloride

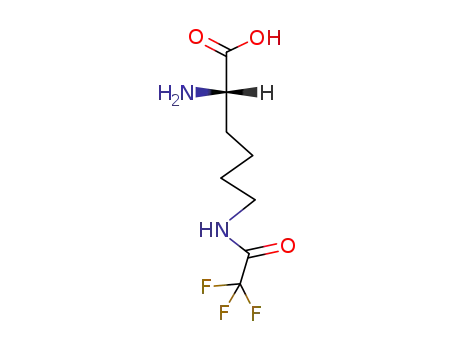
(S)-6-trifluoroacetylamino-2-aminohexanoic acid
| Conditions | Yield |
|---|---|
|
With sodium hydroxide;
|
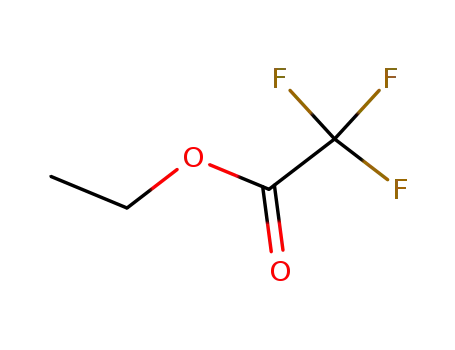
ethyl trifluoroacetate,


L-Lysine hydrochloride


(S)-6-trifluoroacetylamino-2-aminohexanoic acid
| Conditions | Yield |
|---|---|
|
With sodium; In ethanol; at 5 - 20 ℃;
|
62% |
|
L-Lysine hydrochloride; With ethanol; sodium;
ethyl trifluoroacetate,; In ethanol; at 20 ℃; Cooling with ice;
With acetic acid; In ethanol;
|
62% |
|
L-Lysine hydrochloride; With sodium; In ethanol; for 1h;
ethyl trifluoroacetate,; at 5 - 20 ℃; for 3h;
With acetic acid; for 0.166667h;
|
56% |

S-ethyl trifluoroacetate

L-Lysine hydrochloride

ethyl trifluoroacetate,
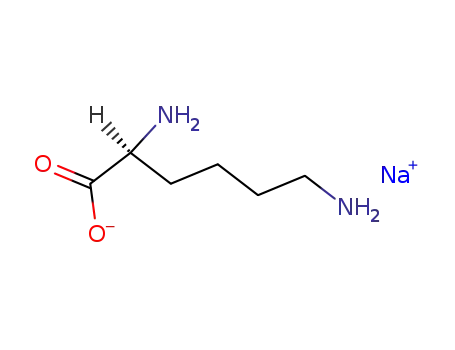
sodium salt of L-lysine
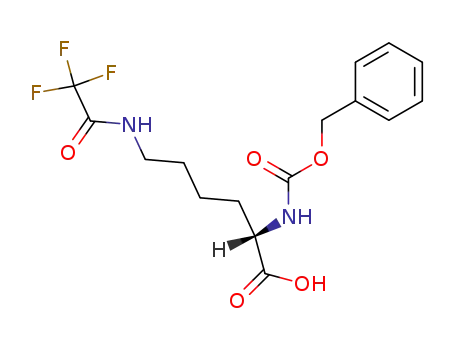
(2S)-2-{[(benzyloxy)carbonyl]amino}-6-[(trifluoroacetyl)amino]hexanoic acid
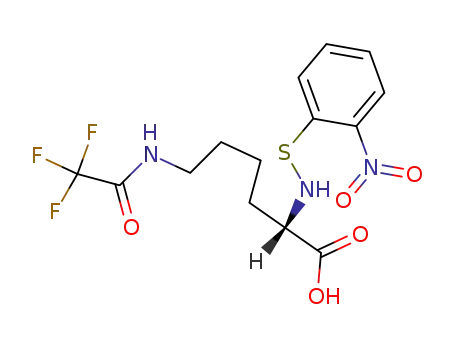
Nα-(o-nitrophenylsulfenyl)-Nε-(trifluoroacetyl)lysine
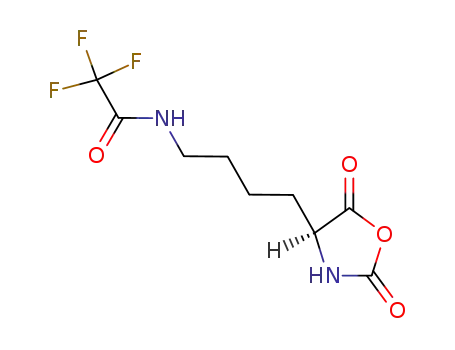
Nε-trifluoroacetyl-L-lysine Nα-carboxanhydride
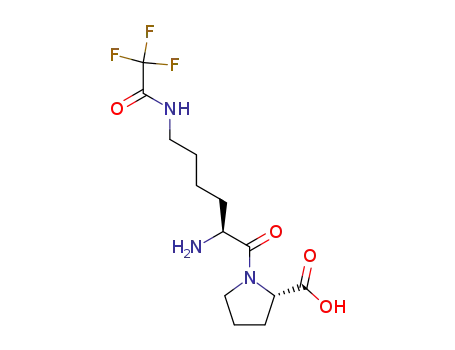
Nε-(trifluoroacetyl)-L-lysyl-L-proline
CAS:56-12-2
CAS:7531-52-4
CAS:556-50-3
CAS:56265-06-6