Your Location:Home >Products >135206-76-7

Product Details
|
Uses |
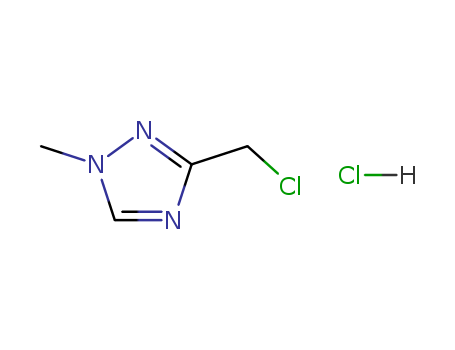
3-(chloromethyl)-1-methyl-1H-1,2,4-triazole hydrochloride is a useful research chemical. |
InChI:InChI=1/C4H6ClN3/c1-8-3-6-4(2-5)7-8/h3H,2H2,1H3
Derivatives of purine, 3H-imidazo[4,5-b]...
Compounds according to Formula (I): or a...
In an effort to find potent antifungal a...
The syntheses of five thiols, including ...
![1-methyl-1H-[1,2,4]-(triazol-5-yl)methanol](/upload/2023/6/0290188b-6ca4-45b8-8ebe-a4628d34328e.png)
1-methyl-1H-[1,2,4]-(triazol-5-yl)methanol

![3-chloromethyl-2-methyl-2H-[1,2,4]triazole hydrochloride](/upload/2023/6/ffd7439d-4187-4bf9-8c95-951077d8b321.png)
3-chloromethyl-2-methyl-2H-[1,2,4]triazole hydrochloride
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
for 0.333333h;
Yield given;
Heating;
|
|
|
In
thionyl chloride;
|
|
|
With
thionyl chloride;
In
dichloromethane;
|
|
|
With
thionyl chloride;
at 0 - 80 ℃;
|
![(1-methyl-1H-[1,2,4]-triazol-3-yl)methanol](/upload/2023/6/367b7549-a182-4387-a614-a1df6d55a135.png)
(1-methyl-1H-[1,2,4]-triazol-3-yl)methanol

![3-chloromethyl-1-methyl-1H-[1,2,4]triazole hydrochloride](/upload/2023/6/59fc2637-c7a5-4b74-a7b0-3790d3d1f5cb.png)
3-chloromethyl-1-methyl-1H-[1,2,4]triazole hydrochloride
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
for 0.666667h;
Heating;
|
90% |
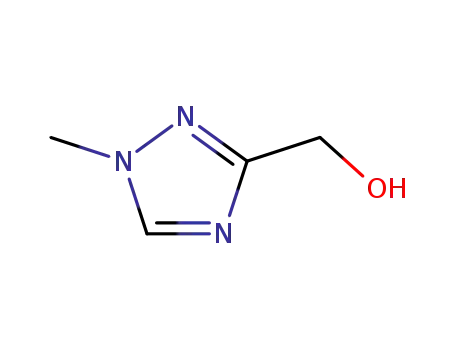
(1-methyl-1H-[1,2,4]-triazol-3-yl)methanol
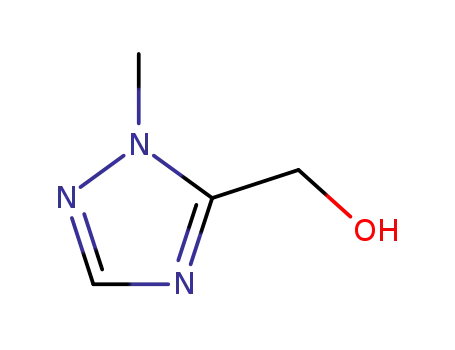
1-methyl-1H-[1,2,4]-(triazol-5-yl)methanol
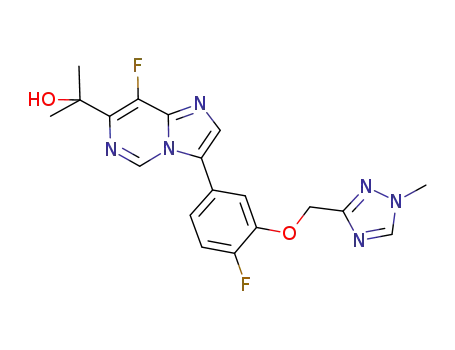
2-{8-fluoro-3-[4-fluoro-3-(1-methyl-1H-[1,2,4]triazol-3-ylmethoxy)phenyl]imidazo[1,2-c]pyrimidin-7-yl}propan-2-ol
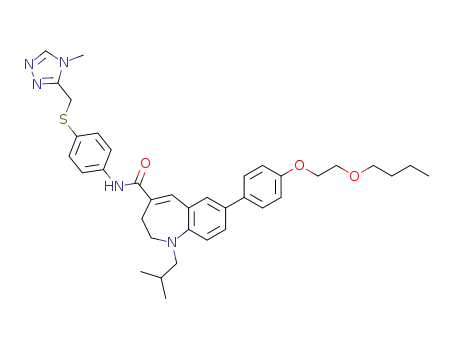
7-{4-[2-(butoxy)ethoxy]phenyl}-1-isobutyl-N-({4-[(4-methyl-4H-1,2,4-triazol-3-yl)methyl]sulfanyl}phenyl)-2,3-dihydro-1H-1-benzazepine-4-carboxamide
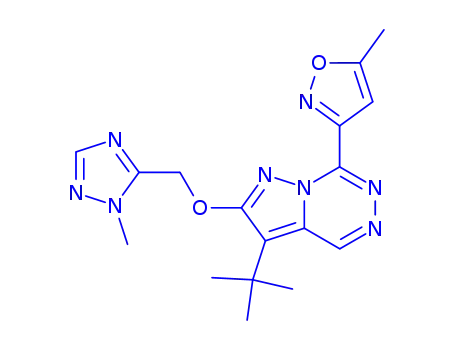
3-tert-butyl-7-(5-methylisoxazol-3-yl)-2-(1-methyl-1H-1,2,4-triazol-5-ylmethoxy)pyrazolo[1,5-d][1,2,4]triazine
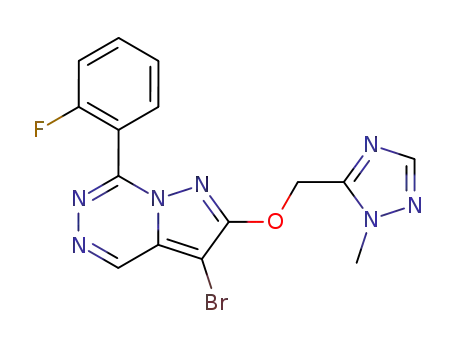
3-bromo-7-(2-fluorophenyl)-2-(2-methyl-2H-[1,2,4]triazol-3-ylmethoxy)pyrazolo[1,5-d][1,2,4]triazine
CAS:138-15-8
CAS:86028-91-3
CAS:5680-79-5
CAS:55819-71-1