Your Location:Home >Products >Biochemical Engineering >5680-79-5
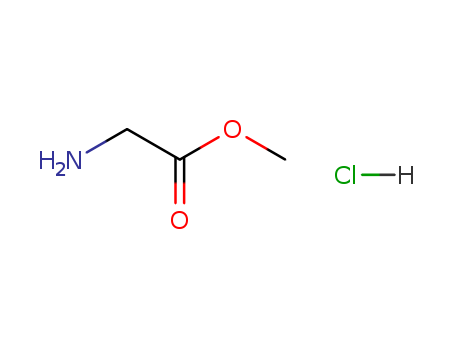

Product Details
|
Chemical Properties |
Crystalline |
|
Uses |
A Glycine ester, a non-essential amino acid for human development. |
|
Purification Methods |
Crystallise the ester salt from MeOH. [Werbin & Spoerri J Am Chem Soc 69 1682 1947, Beilstein 4 H 340, 4 III 1116.] |
InChI:InChI=1/C3H7NO2/c1-6-3(5)2-4/h2,4H2,1H3/p+1
A method for preparing N-(imidomethyl)gl...
The carboxylic group responsible for the...
A novel and selective sorbent for micro-...
Two different types of new phosphinamide...
The entry of enveloped virus requires th...
Catalytic transformation of alcohols via...
A silver-mediated [3 + 2] cycloaddition ...
methanol

glycine

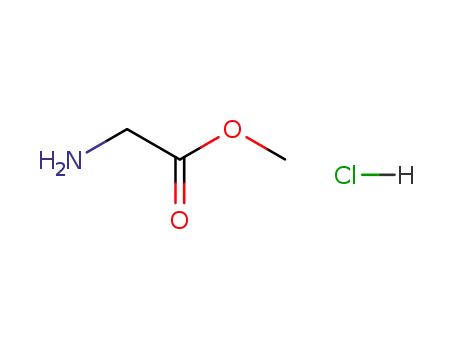
glycine ethyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
With
acetyl chloride;
at 0 - 20 ℃;
|
100% |
|
methanol;
With
thionyl chloride;
for 1h;
Cooling with ice;
glycine;
at 20 - 66 ℃;
for 6.5h;
|
100% |
|
With
thionyl chloride;
at 0 ℃;
Reflux;
Inert atmosphere;
|
100% |
|
With
thionyl chloride;
at 0 - 20 ℃;
|
100% |
|
glycine;
With
chloro-trimethyl-silane;
at 20 ℃;
for 0.166667h;
methanol;
at 20 ℃;
for 48h;
|
100% |
|
With
thionyl chloride;
for 3h;
Reflux;
|
100% |
|
With
thionyl chloride;
at -5 - 80 ℃;
for 3h;
|
100% |
|
With
thionyl chloride;
at 25 - 30 ℃;
for 0.5h;
microwave irradiation;
|
99% |
|
With
thionyl chloride;
at -5 - 20 ℃;
for 3h;
Inert atmosphere;
|
99% |
|
With
thionyl chloride;
at 40 ℃;
for 2h;
|
98% |
|
With
thionyl chloride;
Heating;
|
98% |
|
With
thionyl chloride;
at -5 - 20 ℃;
|
98% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 6h;
|
98% |
|
With
thionyl chloride;
for 8h;
Heating;
|
97% |
|
With
thionyl chloride;
for 8h;
Reflux;
|
97% |
|
With
thionyl chloride;
at 0 - 20 ℃;
|
97% |
|
With
chloro-trimethyl-silane;
at 20 ℃;
for 24h;
|
96% |
|
With
thionyl chloride;
at 20 ℃;
Inert atmosphere;
|
96% |
|
With
thionyl chloride;
at 0 - 20 ℃;
|
96% |
|
With
thionyl chloride;
at 0 - 60 ℃;
for 4h;
|
95% |
|
With
hydrogenchloride;
at 20 - 100 ℃;
for 3.08333h;
Temperature;
|
95.64% |
|
With
hydrogenchloride;
In
water;
at 40 - 60 ℃;
Large scale;
|
95.28% |
|
With
hydrogenchloride;
at 20 ℃;
for 18h;
|
94% |
|
With
acetyl chloride;
at 0 ℃;
for 3h;
Reflux;
|
92% |
|
With
thionyl chloride;
at 16 - 20 ℃;
for 48h;
|
91.5% |
|
With
chloro-trimethyl-silane;
at 20 ℃;
for 24h;
Sealed tube;
|
90% |
|
With
chloro-trimethyl-silane;
at 20 ℃;
for 24h;
Sealed tube;
|
90% |
|
With
hydrogenchloride;
for 2h;
Reflux;
|
90% |
|
methanol;
With
acetyl chloride;
at -5 - 5 ℃;
for 1h;
glycine;
at 70 ℃;
for 5h;
|
90.68% |
|
With
thionyl chloride;
at 0 ℃;
Reflux;
|
90% |
|
With
thionyl chloride;
at 0 - 20 ℃;
|
89% |
|
With
thionyl chloride;
|
89% |
|
With
thionyl chloride;
In
methanol;
for 0.5h;
Heating;
|
88% |
|
With
thionyl chloride;
at 0 ℃;
Reflux;
|
88% |
|
With
thionyl chloride;
at -5 ℃;
for 7h;
Reflux;
|
88% |
|
With
thionyl chloride;
at 0 - 20 ℃;
Inert atmosphere;
|
84% |
|
With
thionyl chloride;
at 0 - 20 ℃;
for 16h;
|
81% |
|
With
thionyl chloride;
7 h, 0-5 deg C; 12 h, 10 deg C; 10 h, 40 deg C;
|
80% |
|
With
hydrogenchloride;
Inert atmosphere;
|
80% |
|
With
thionyl chloride;
at 0 - 20 ℃;
|
80% |
|
With
hydrogenchloride;
at 0 ℃;
|
80% |
|
With
thionyl chloride;
at 40 ℃;
for 2h;
|
73% |
|
With
thionyl chloride;
for 2h;
Heating;
|
|
|
With
thionyl chloride;
Ambient temperature;
|
|
|
With
thionyl chloride;
Heating;
|
|
|
With
thionyl chloride;
for 6h;
Ambient temperature;
|
|
|
With
thionyl chloride;
for 1h;
Heating;
|
|
|
With
hydrogenchloride; 3 A molecular sieve;
for 2.5h;
Heating;
|
|
|
With
thionyl chloride;
Yield given;
1) RT, 30 min, 2) 40 deg C, 2 h;
|
|
|
With
hydrogenchloride;
for 4h;
Yield given;
Heating;
|
|
|
With
thionyl chloride;
for 24h;
Yield given;
Ambient temperature;
|
|
|
With
thionyl chloride;
|
|
|
With
hydrogenchloride;
|
|
|
With
thionyl chloride;
|
|
|
methanol;
With
thionyl chloride;
at 0 ℃;
for 0.5h;
glycine;
Heating;
|
|
|
With
thionyl chloride;
for 6h;
|
|
|
With
hydrogenchloride;
for 2h;
Heating / reflux;
|
|
|
With
sulfuryl dichloride;
at 0 ℃;
|
|
|
With
thionyl chloride;
at 60 - 70 ℃;
for 10h;
Heating;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
|
|
|
With
hydrogenchloride;
|
|
|
With
thionyl chloride;
at 20 ℃;
|
|
|
With
thionyl chloride;
at 0 ℃;
Reflux;
|
|
|
With
hydrogenchloride;
|
|
|
With
thionyl chloride;
at 78 ℃;
|
|
|
With
thionyl chloride;
at 60 - 70 ℃;
|
|
|
With
acetyl chloride;
Reflux;
|
|
|
With
thionyl chloride;
at 55 ℃;
for 6h;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
at 5 ℃;
Inert atmosphere;
Reflux;
|
|
|
With
thionyl chloride;
In
methanol;
Reflux;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
at 78 ℃;
|
|
|
With
thionyl chloride;
at 0 ℃;
for 1.5h;
Inert atmosphere;
Reflux;
|
|
|
With
thionyl chloride;
at -5 - 20 ℃;
|
|
|
With
thionyl chloride;
Inert atmosphere;
Reflux;
|
|
|
With
thionyl chloride;
|
|
|
methanol;
With
thionyl chloride;
at 0 ℃;
for 1h;
glycine;
at 90 ℃;
for 15h;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
|
|
|
methanol;
With
thionyl chloride;
In
methanol;
at 0 - 20 ℃;
for 2.5h;
glycine;
In
methanol;
for 4h;
Reflux;
|
|
|
methanol;
With
thionyl chloride;
at 0 - 20 ℃;
glycine;
for 4h;
Reflux;
|
|
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
at 0 ℃;
for 4h;
Reflux;
|
1.253 g |
|
methanol; glycine;
With
thionyl chloride;
at 0 ℃;
Heating;
|
|
|
With
thionyl chloride;
at 0 - 20 ℃;
for 16h;
Inert atmosphere;
|
|
|
With
thionyl chloride;
at 0 ℃;
Reflux;
|
|
|
With
thionyl chloride;
at 60 ℃;
Cooling with ice;
|
|
|
With
hydrogenchloride;
at 65 - 70 ℃;
for 1.25h;
|
4.93 g |
|
With
thionyl chloride;
|
|
|
With
thionyl chloride;
at 20 ℃;
Cooling with ice;
|
|
|
With
thionyl chloride;
at 0 - 20 ℃;
|
|
|
With
thionyl chloride;
at -5 - 20 ℃;
for 3h;
Inert atmosphere;
|
|
|
With
thionyl chloride;
for 12h;
Reflux;
|
|
|
glycine;
With
chloro-trimethyl-silane;
at 20 ℃;
for 0.333333h;
methanol;
at 20 ℃;
for 24h;
|
|
|
With
thionyl chloride;
at 0 ℃;
|
|
|
With
thionyl chloride;
at 0 ℃;
Reflux;
Inert atmosphere;
Sealed tube;
|
|
|
With
thionyl chloride;
at 0 - 20 ℃;
Inert atmosphere;
|
|
|
With
thionyl chloride;
at -10 - 20 ℃;
for 0.6h;
|
|
|
With
thionyl chloride;
at 0 - 65 ℃;
for 8h;
Inert atmosphere;
|
16.57 g |
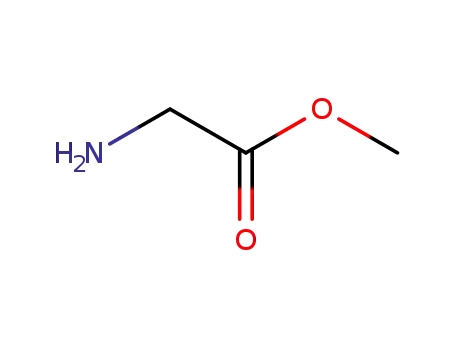
methoxycarbonylmethylamine


glycine ethyl ester hydrochloride
| Conditions | Yield |
|---|---|
|
With
thionyl chloride;
In
methanol;
at -10 ℃;
for 0.166667h;
|
99.5% |

methanol
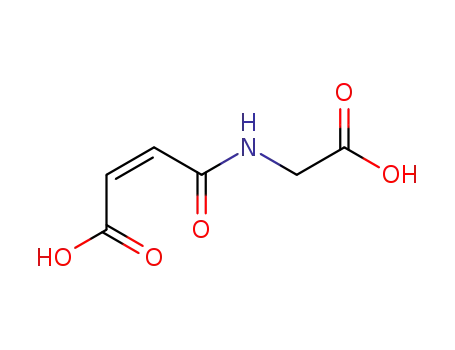
(Z)-4-((carboxymethyl)amino)-4-oxobut-2-enoic acid
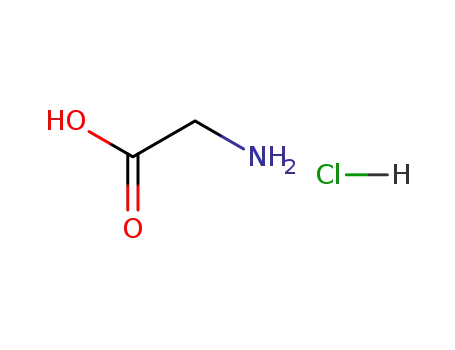
2-aminoethanoic acid hydrochloride
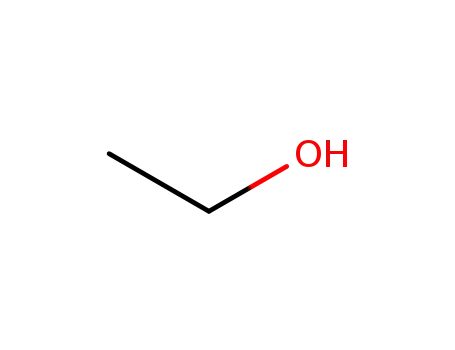
ethanol
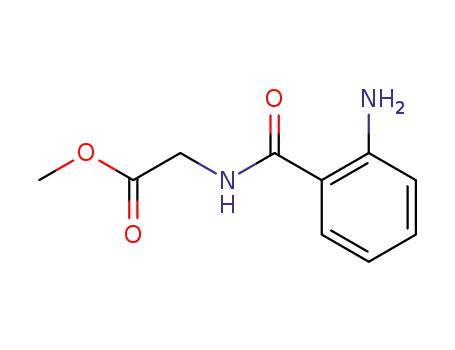
2-amine-N-(methoxycarbonylmethyl)benzamide
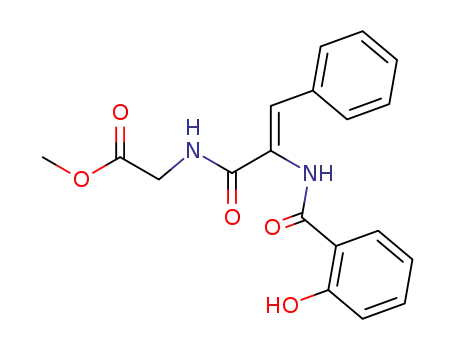
N-(α-salicyloylamino-trans-cinnamoyl)-glycine methyl ester
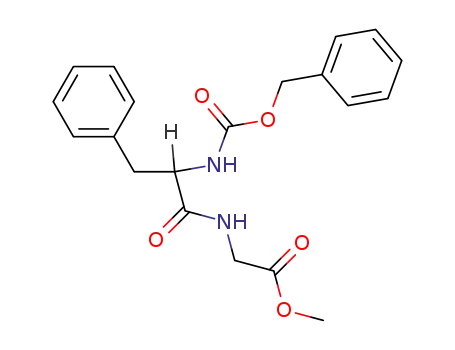
N-(N-benzyloxycarbonyl-DL-phenylalanyl)-glycine methyl ester
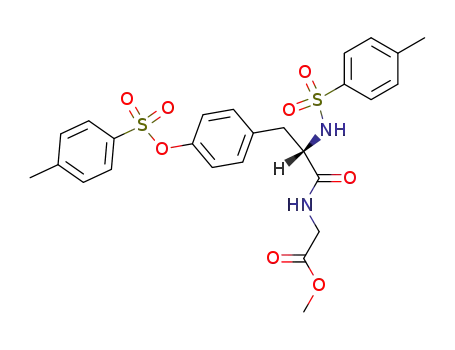
N-[N,O-bis-(toluene-4-sulfonyl)-L-tyrosyl]-glycine methyl ester
CAS:138-15-8
CAS:86028-91-3
CAS:27532-96-3
CAS:135206-76-7